Another win for antibody-drug conjugates in breast cancer
- Sacituzumab govitecan, an antibody-drug conjugate targeting Trop-2 antigen, was reported to increase survival in HR-positive, EGFR2-negative breast cancer.
- This is yet another success for ADCs, which validates the concept of bringing together monoclonal antibodies and cytotoxic molecules for cancer treatment.
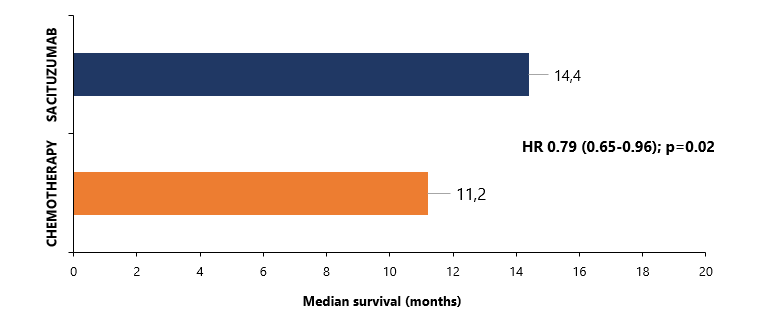
The results of the pivotal efficacy trial of sacituzumab govitecan, an innovative antibody-drug conjugate developed by Gilead Sciences, have just been published in The Lancet, showing improved survival of HR-positive, EGFR2-negative breast cancer patients receiving the new drug1. In this trial, 543 subjects were randomized to treatment with sacituzumab govitecan versus standard chemotherapy. The overall survival was 14.4 months in the former group and only 11.2 months in the latter, for a hazard ratio of 0.79 [0.65-0.96, p=0.02). Moreover, the tested drug boosted the response rate from 14% to 21% and increased time to deterioration of health status and quality of life. The only downside was a higher frequency of severe and serious adverse events (74% vs 60% and 28% vs 19%, respectively), although the safety profile was consistent with the previous studies.
Figure 1 Overall survival in patients randomized to sacituzumab versus chemotherapy (TROPiCS-02)
Sacituzumab govitecan is a conjugate of humanized monoclonal antibody targeting Trop-2 antigen with SN-38, which is an active metabolite of already approved anti-cancer drug, irinotecan. Activation of Trop-2 receptor leads to enhanced growth and spread of cancer cells, and therefore is regarded as an attractive treatment target. As with other ADCs, the conjugated small molecule is too toxic to be administered directly to cancer patients.
The reported results mark another step forward for ADCs to become a treatment of choice in various oncology indications. The prolonged survival obtained with sacituzumab govitecan unequivocally shows the added benefit of conjugated antibodies, which has previously been shown with trastuzumab emtansine and trastuzumab deruxtecan. Further ADCs for breast cancer are currently in development, for example trastuzumab duocarmazine and dapotamab deruxtecan.
- Rugo et al. “Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial”. The Lancet; 23 August 2023.doi: https://doi.org/10.1016/S0140-6736(23)01245-X ↩︎