COVID-19 vaccine for 2024/25 season will target JN.1 variant
- In face of the continuing evolution of SARS-CoV-2 virus, WHO and EMA published a recommendation to update the composition of COVID-19 vaccines for 2024/25 season to JN.1 variant.
- In recent months JN.1 has become the most widely circulating variant worldwide. It differs from the XBB variant included in the current season vaccines, causing substantial fall in their effectiveness.
- WHO admitted that there are many uncertainties surrounding the vaccine update recommendation, including the likely appearance of the new, more drifted variants.
In response to the evolving landscape of SARS-CoV-2 variants, the European Medicines Agency (EMA) and the World Health Organization (WHO) have recommended updates to the composition of COVID-19 vaccines for the upcoming 2024/25 season. As of April 2024, nearly all circulating SARS-CoV-2 variants reported in publicly available databases are JN.1-derived variants, which are antigenically distinct from the previously circulating viruses. This antigenic drift caused significant decline in the protection afforded by the XBB vaccines used in 2023 winter campaigns. One study performed in the U.S. reported 50%-61% effectiveness during the XBB-like variant predominance falling to only 24%-35% after the emergence of JN.1 (although effectiveness against severe outcomes can be much higher). Other investigations returned similar results.
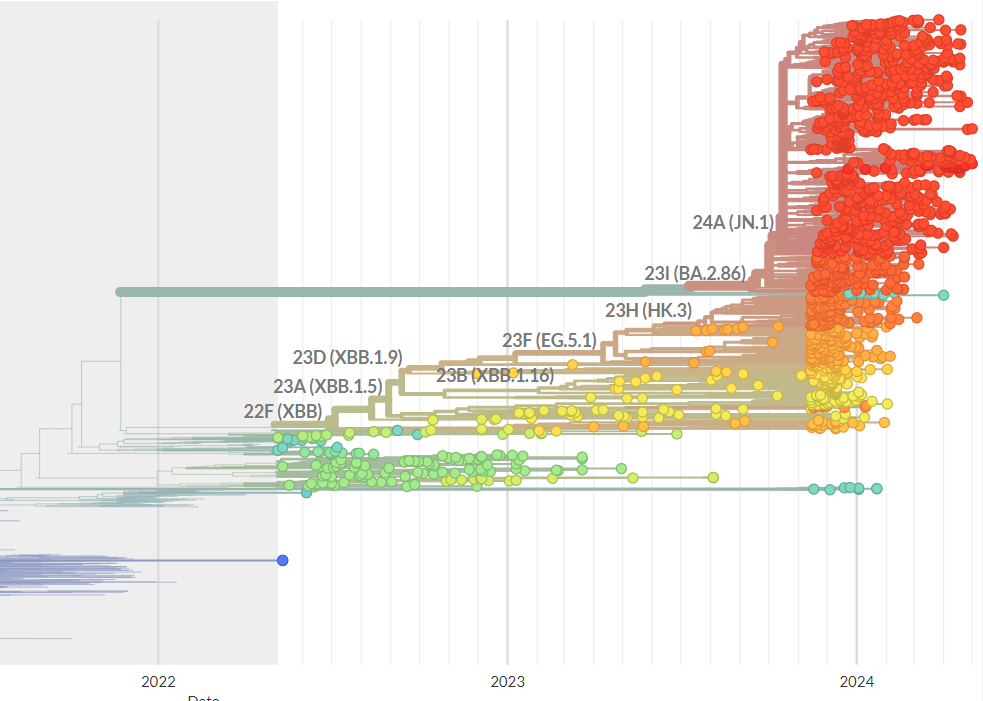
The EMA and WHO have underscored the importance of tailoring future COVID-19 vaccine formulations to target the newly emerging lineages and ensure adequate protection of the most vulnerable populations. However, at the same time they acknowledged important limitations in the available data, which may lead to inaccurate recommendations. After all, many novel variants may emerge in the long time interval between the publication of EMA/WHO advice and initiation of vaccination campaigns, leading to significant mismatch between the vaccine antigen and circulating virus. The same phenomenon is frequently observed in influenza vaccines.
Some vaccine manufacturers are already well prepared to reformulate their products. Right after EMA/WHO recommendation, Novavax announced that its JN.1 variant vaccine candidate is already in development, showing promising non-clinical data. The new formulation was capable of inducing high neutralization titres in animals against all major JN variants, while also producing polyfunctional cellular immunity responses. Novavax declared readiness to deliver the adapted vaccines globally this fall.
The COVID-19 is going to stay with us for good and we clearly need better solutions to protect the frailest populations against its dire consequences. Even with full antigenic match, effectiveness of the current vaccines ranges from 40% to 70%, which is much lower than for earliest formulations (>90%). Scientists, pharmaceutical companies and regulatory agencies all over the world are working on new vaccine formulations, testing different platforms, adjuvants or administration routes. One of the most interesting candidates are live vaccines administered intranasally, which elicit higher mucosal immunity and engage different immune mechanisms than mRNA or protein vaccines. However, it is clear that these innovative solutions won’t be ready before the upcoming season. Therefore, we must rely on currently available options which, although not offering complete protection, remain our most effective preventive measures against COVID-19.
Prepared by:
Sources and further reading
- European Medicines Agency (EMA). “ETF recommends updating COVID-19 vaccines to target new JN.1 variant” (April 30, 2024). Link: https://www.ema.europa.eu/en/news/etf-recommends-updating-covid-19-vaccines-target-new-jn1-variant.
- World Health Organization (WHO). Statement on the antigen composition of COVID-19 vaccines (April 26, 2024). Link: https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-composition-of-covid-19-vaccines.
- Caffrey, Aisling, et al. “Effectiveness of BNT162b2 XBB vaccine in the US Veterans Affairs Healthcare System.” medRxiv (2024): 2024-04.
- Novavax Press Release “Novavax prepared to deliver protein-based non-mRNA JN-1 COVID-19 vaccine in line with WHO recommendations this fall.” Link: https://ir.novavax.com/press-releases/Novavax-Prepared-to-Deliver-Protein-based-Non-mRNA-JN-1-COVID-19-Vaccine-in-Line-with-WHO-Recommendation-this-Fall