Top 5 most expected biologics in 2024
Biologics, Drug development, EMA, FDA, Regulatory

- A while ago we reviewed the top 10 biologics approved in 2023 by the EMA and/or FDA. In this article we decided to take a closer look at most groundbreaking biologics scheduled for 2024 approval.
- Five candidates were selected for review based on their commercial potential or anticipated medical impact: donanemab, sotatercept, dapotamab deruxtecan, Anktiva and mRNA-1345 vaccine. Sotatercept, Anktiva and mRNA-1345 have already received approval from the FDA; two other are still awaiting regulatory decisions.
- The expansion and diversification of biologics market witnessed over the recent decades will likely continue into 2024. Time will tell if the last year’s record of 34 FDA approvals will be beaten.
Last year, we witnessed the approval of several groundbreaking biologics, some belonging to the emerging classes of bispecific antibodies or CAR-T therapies. Reflecting recent trends, the number and proportion of approved biologic drugs reached an all-time high, with 18 new products approved in the European Union and 34 in the United States. This rapid growth in the biologics market will certainly continue, with an increasing share of drugs showcasing innovative structures or unique mechanisms of action.
Earlier, we published a review of the top biologics approved in 2023. This list included several bispecific antibodies (talquetamab, glofitamab, elranatamab), CAR-T therapies (delandistrogene moxeparvovec, Examgamglogene autotemcel), mRNA vaccines (Abrysvo and Arexvy) and conventional monoclonal antibodies (nirsevimab, lecanemab, rozanolixizumab and ublituximab) – all with a huge potential to become a new blockbuster and/or revolutionize medical care. Now we decided that before writing our next top 10 article in 2025, we will do a little spoiler and highlight the most anticipated biologics scheduled for approval this year. Using the same criteria as our previous publication, we have selected the following five molecules:
- Donanemab (Eli Lilly)
- Sotatercept (Merck)
- Dapotamab deruxtecan (AstraZeneca)
- mRNA-1345 (Moderna)
- Anktiva (ImmunityBio)
As we are already well into 2024, three of these candidates (sotatercept, mRNA-1345, and Anktiva) have received FDA nod, with the remaining two still awaiting regulatory decisions. However, given the positive findings from preclinical and clinical studies, their authorization seems to be a matter of time.
Donanemab (Eli Lilly)
Donanemab is a monoclonal antibody targeting amyloid beta (Aβ), an abnormal protein associated with Alzheimer’s disease (AD) pathogenesis.1–3 Like lecanemab, which was approved last year by the FDA for early AD treatment, donanemab has shown promise in Phase II/III trials, demonstrating an ability to slow disease progression in early symptomatic patients. However, there is an ongoing discussion in the field regarding its clinical significance.4–6
The delay in AD progression comes at the cost of potentially serious adverse reactions, particularly ARIA – Amyloid-Related Imaging Abnormalities. ARIA can manifest as brain edema or cerebral hemorrhage, and is thought to be triggered by an antibody-induced inflammatory response (mechanism is still incompletely understood).7 Approximately 6% of patients in clinical trials reported symptomatic ARIA, raising concerns about the drug’s safety given its modest short-term benefits.4,5 Consequently, some experts question donanemab’s utility and caution against its overprescription.8
The donanemab’s road to regulatory approval has so far been very bumpy. The FDA initially declined the accelerated approval pathway due to an insufficient number of patients with 12+ months of follow-up. Eli Lilly refiled their biologics application in the summer of 2023 with anticipated decision in Q1 2024. Unfortunately, the FDA has recently announced a delay in approval, without disclosing specific reasons.9 Despite numerous setbacks, the game is certainly worth a candle. Nearly 7 million people in the US alone suffer from Alzheimer’s disease, a number that continues to rise annually.10 Donanemab’s revenue is projected to reach $4.88 billion by 2036.11 However, the Eli Lilly’s product will face intense competition from the already licensed lecanemab, which will have had more time to establish a strong market position.
Sotatercept (Merck)
Sotatercept, developed by Acceleron Pharma and now under Merck, is a groundbreaking fusion protein designed to treat pulmonary arterial hypertension (PAH). PAH symptoms include breath shortness, chest pain, fatigue, leg edema and fainting, which can be severe and even life-threatening.
Sotatercept works by inhibiting activin – a small signaling protein, that promotes vascular obliteration in the lungs. The drug consists of the Fc domain of immunoglobulin and extracellular domain of activin type 2 receptor, which traps activin molecules and prevents them from interacting with their natural receptors expressed throughout the pulmonary vascular bed.
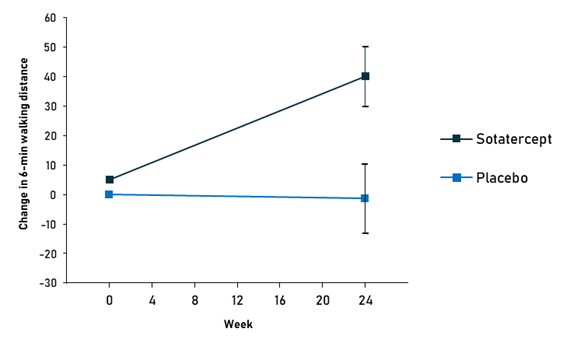
The drug has successfully passed the consecutive phases of clinical development, demonstrating the ability to increase exercise capacity and reduce the risk of serious cardiovascular events.12,13 In the pivotal phase III trial, patients treated with sotatercept increased their 6-minute walking distance by 34.4 m, compared to a 1-meter deterioration in the placebo group. Nine out of 163 (5.5%) patients on sotatercept experienced clinical worsening or death compared to 42 (26.2%) patients on placebo.13
Sotatercept was the lead molecule of Acceleron Pharma, acquired by Merck in early 2021. Now branded WINREVAIR®, it received FDA approval on March 26th as a first-in-class treatment for adults with PAH.14 Its sales are projected to reach $2 billion by 2028, highlighting its significant potential in the market.15
Datopotamab deruxtecan (Daiichi Sankyo/AstraZeneca)
Antibody-drug conjugates (ADCs) are gaining more and more recognition as highly effective cancer drugs, as study after study reports improved survival rates in comparison to the standard monoclonals or chemical drugs. ADCs, combining the power of specific monoclonal antibodies and highly cytotoxic molecules precisely delivered to cancer cells, allow to achieve exceptional efficacy against many advanced tumors, while maintaining manageable toxicity levels.16 Such feat would never be possible with their separate administration. The EMA and FDA have already approved several ADCs, with trastuzumab emtansine and trastuzumab deruxtecan standing out as particularly successful. Both are used for treating breast cancer patients who have developed resistance to the unconjugated parental antibody (trastuzumab, also known as Herceptin).17,18
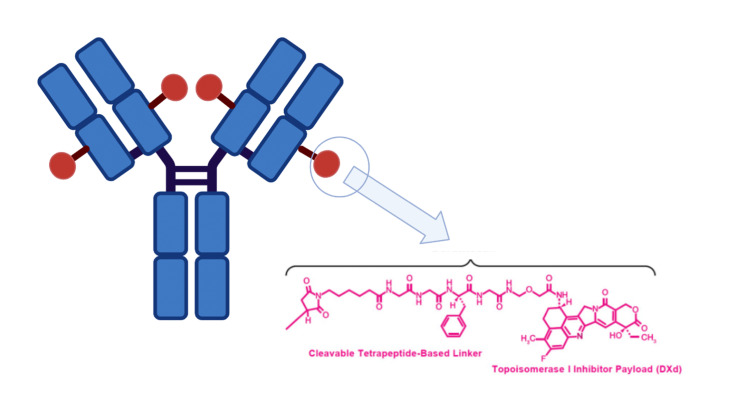
Datopotamab deruxtecan (Dato-DXd) is a promising investigational ADC composed of humanized monoclonal antibody targeting TROP2, linked to a number of topoisomerase I inhibitor payloads.20 TROP2 is a tumor-associated calcium signal transducer, believed to play a crucial role in cancer progression by activating various signaling pathways.21 Its elevated expression is detected in several cancers including colorectal, lung and breast cancer.
The results of the first pivotal studies with Dato-DXd are slowly trickling in, showing improved progression-free survival (PFS) compared to the standard chemotherapy.20,22 One of such studies is TROPION-Lung01, which demonstrated the superiority of the new ADC in patients with metastatic non-small cell lung cancer (NSCLC), with a median PFS of 4.4 months on Dato-DXd versus 3.7 months on control regimen (docetaxel).22
Dato-DXd is the product of fruitful cooperation between AstraZeneca and Daiichi Sankyo, which teamed several years ago to advance the development of new antibody-drug conjugates.23 The companies are expected to file marketing authorization application still this year, with a probable launch in early 2025.24
mRNA-1345 (Moderna)
Another vaccine against respiratory syncytial virus (RSV) is entering the biologics market in 2024, joining the ranks of already approved products from Pfizer and GSK.25 This new contender, mRNA-1345, developed by Moderna, is notable for being the second vaccine after COVID-19 to employ the groundbreaking mRNA technology. Like its competitors, mRNA-1345 is based on RSV fusion (F) glycoprotein stabilized in pre-fusion conformation. Encapsulated in the same liponanoparticles as COVID-19 mRNA vaccines, it is capable of eliciting high titres of neutralizing antibodies against both RSV types A and B.26
In the main safety and efficacy trial involving elderly subjects, mRNA-1345 was found to reduce the risk of primary endpoint (RSV-associated lower respiratory tract disease with 2 or more symptoms) by 83.7% (66.0%, 92.2%).27 Importantly, none of the patients in vaccine group had medically attended RSV disease compared to 5 in the placebo group. mRNA-1345 was safe, with only a small percentage of subjects reporting transient severe local and systemic reactions. The incidence of serious adverse events and deaths was similar in both study arms.27
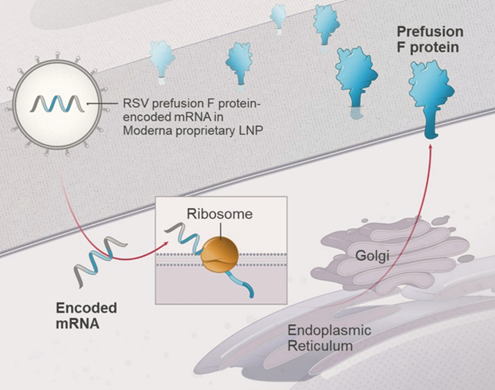
When writing this article, mRNA-1345 received FDA approval for the prevention of lower respiratory tract disease caused by RSV in adults aged 60 years and older.25 The vaccine will be marketed under the brand name mRESVIA. Moderna has also submitted marketing authorization applications to the EU, Swiss and Australian drug authorities – as of the publication date, the vaccine is still in the review process.
As a significant part of the population is eligible for RSV vaccination, Moderna’s product is expected to generate significant profits, potentially reaching an annual total of $1.41 bln by 2036 in the US alone.29 These forecasts are optimistic despite the fact that mRNA-1345 joins the party one year after the two major competitors: GSK’s Arexvy and Pfizer’s Abrysvo, which use the recombinant protein technology. Only in the third quarter of 2023, Arexvy and Abrysvo generated revenues of ~$900 mln and $375 mln, respectively, underscoring the high business value of RSV vaccines.30,31
Anktiva (ImmunityBio)
Anktiva is a first-in-class IL-15 superagonist characterized by a highly innovative structure. It consists of a fusion protein, combining IL-15 receptor domain (IL-15RαSu) with Fc part of IgG1, which is additionally complexed with an IL-15 mutant.32 Anktiva works by activating NK cells and cytotoxic lymphocytes, and is intended for use in patients with unresponsive solid tumors such as invasive bladder cancer. For more information on this product, refer to our previous article: “First-in-class IL-15 superagonist wins FDA approval”.
Having already launched in the US, Anktiva’s full commercial potential remains untapped as pivotal studies for other indications are still underway.33 ImmunityBio anticipates realizing this potential fully in the coming years.
Conclusions
The year 2024 is brimming with innovative biologics, each presenting high chances of becoming the next blockbuster. Projected sales of these drugs over the coming years are counted in billions of dollars. However, their financial prospects are not the sole reason for including them among the most expected biologics drugs in 2024. These five molecules are remarkable for many other reasons: some are first-in-class, other have unique structural features or unusual pharmacological properties.
Undoubtedly, many other biologics will gain approval in the second half of 2024, some of which may surpass our selected drugs in terms of commercial success and/or medical impact. Early next year, we will publish a summary of the top achievements of 2024, just as we did for 2023. This will allow us to check the accuracy of our selection of the five most promising drugs.
FAQ
Prepared by:

Adam Tuszyner
Regulatory Compliance Specialist
References
- Harris, Emily. “Alzheimer drug lecanemab gains traditional FDA approval.” JAMA (2023).
- Tuszyner, Adam “Top 10 biologics approved in 2023”. Mabion.eu (March 14, 2024). Available at: https://www.mabion.eu/science-hub/articles/top-10-biologics-approved-in-2023/
- Rashad, Areeba, et al. “Donanemab for Alzheimer’s disease: a systematic review of clinical trials.” Healthcare. Vol. 11. No. 1. MDPI, 2022.
- Mintun, Mark A., et al. “Donanemab in early Alzheimer’s disease.” New England Journal of Medicine 384.18 (2021): 1691-1704.
- Sims, John R., et al. “Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial.” Jama 330.6 (2023): 512-527.
- Tarawneh, Rawan, and Vernon S. Pankratz. “The search for clarity regarding “clinically meaningful outcomes” in Alzheimer disease clinical trials: CLARITY-AD and Beyond.” Alzheimer’s Research & Therapy 16.1 (2024): 37.
- Hampel, Harald, et al. “Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics.” Brain 146.11 (2023): 4414-4424.
- Kurkinen M. Lecanemab (Leqembi) is not the right drug for patients with Alzheimer’s disease. Adv Clin Exp Med. 2023;32(9):943–947. doi:10.17219/acem/171379.
- Radcliffe, Shawn “FDA delays approval of Eli Lilly Alzheimer’s drug.” Healthline (March 11, 2024). Available at: https://www.healthline.com/health-news/fda-delays-approval-eli-lilly-alzheimers-drug.
- Alzheimer’s Association “Alzheimer’s Disease Facts and Figures”. Available at: https://www.alz.org/alzheimers-dementia/facts-figures.
- Pharmaceutical Technology “Risk adjusted net present value: What is the current valuation of Eli Lilly and Co’s Donanemab?” (May 3, 2024). Available at: https://www.pharmaceutical-technology.com/data-insights/donanemab-eli-lilly-and-co-net-present-value/.
- Humbert, Marc, et al. “Sotatercept for the treatment of pulmonary arterial hypertension.” New England Journal of Medicine 384.13 (2021): 1204-1215.
- Hoeper, Marius M., et al. “Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension.” New England Journal of Medicine 388.16 (2023): 1478-1490.
- Merck Press Release “FDA approves Merck’s WINREVAIR (sotatercept-csrk), a first-in-class treatment for adults with pulmonary arterial hypertension” (March 26, 2024). Available at: https://www.merck.com/news/fda-approves-mercks-winrevair-sotatercept-csrk-a-first-in-class-treatment-for-adults-with-pulmonary-arterial-hypertension-pah-who-group-1/.
- Gardner, Jonathan “FDA approves Merck lung disease drug acquired in $11B deal.” BioPharma Dive (March 26, 2024). Available at: https://www.biopharmadive.com/news/merck-winrevair-fda-approval-pah-pulmonary-arterial-hypertension/711149/.
- Birrer, Michael J., et al. “Antibody-drug conjugate-based therapeutics: state of the science.” JNCI: Journal of the National Cancer Institute 111.6 (2019): 538-549-968.
- Peddi, Parvin F., and Sara A. Hurvitz. “Trastuzumab emtansine: the first targeted chemotherapy for treatment of breast cancer.” Future oncology 9.3 (2013): 319-326.
- Andrikopoulou, Angeliki, et al. “Trastuzumab deruxtecan (DS-8201a): the latest research and advances in breast cancer.” Clinical breast cancer 21.3 (2021): e212-e219.
- Schipilliti, Francesca Matilde, et al. “Datopotamab deruxtecan: A novel antibody drug conjugate for triple-negative breast cancer.” Heliyon 10.7 (2024).
- AstraZeneca press release “Datopotamab deruxtecan improved progression-free survival vs. chemotherapy in patients with previously treated non-small cell lung cancer in TROPION-Lung01 Phase III trial” (October 23, 2023). Available at: https://www.astrazeneca.com/media-centre/press-releases/2023/datopotamab-deruxtecan-improved-progression-free-survival-vs-chemotherapy-in-tropion-lung01-phase-iii-trial.html.
- McDougall, Annie RA, et al. “Trop2: from development to disease.” Developmental Dynamics 244.2 (2015): 99-109.
- AstraZeneca press release “Datopotamab deruxtecan showed clinically meaningful overall survival improvement vs. chemotherapy in patients with advanced nonsquamous non-small cell lung cancer in TROPION-Lung01 Phase III trial” (May 27, 2024). Available at: https://www.astrazeneca-us.com/media/press-releases/2024/datopotamab-deruxtecan-showed-clinically-meaningful-overall-survival-improvement-vs-chemotherapy-in-patients-with-advanced-nonsquamous-non-small-cell-lung-cancer-in-tropion-lung01-phase-iii-trial.html.
- AstraZeneca press release “AstraZeneca and Daiichi Sankyo enter collaboration to develop and commercialize new antibody drug conjugate” (July 27, 2020). Available at: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-daiichi-sankyo-enter-collaboration-to-develop-and-commercialise-new-antibody-drug-conjugate.html.
- https://clarivate.com/drugs-to-watch/drugs-to-watch-listing/datopotamab-deruxtecan/
- Moderna News “Moderna Receives U.S. FDA Approval for RSV Vaccine mRESVIA(R)” (May 31, 2024). Available at: https://investors.modernatx.com/news/news-details/2024/Moderna-Receives-U.S.-FDA-Approval-for-RSV-Vaccine-mRESVIAR/default.aspx.
- Moderna presentation for Advisory Committee on Immunization Practices (ACIP) “Overview of Moderna’s Investigational RSV Vaccine (mRNA-1345) in Adults ≥ 60 Years of Age” (Feb 29, 2024). Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/02-RSV-Adults-Das-508.pdf.
- Wilson, Eleanor, et al. “Efficacy and safety of an mRNA-based RSV Pref vaccine in older adults.” New England Journal of Medicine 389.24 (2023): 2233-2244.
- Moderna “Efficacy and Safety of mRNA-1345, an RSV Vaccine, in Older Adults: Results through ≥6 Months of Follow-up and Evaluation of Correlate of Protection Against RSV.” Presented at the 8th ReSViNET (RSVVW 2024) conference (February 15, 2024).
- Pharmaceutical Technology “Risk adjusted net present value: What is the current valuation of Moderna’s MRNA-1345?” (updated May 16, 2024). Available at: https://www.pharmaceutical-technology.com/data-insights/mrna-1345-moderna-net-present-value/?cf-view.
- Alvarado, Delilah “GSK raises forecasts on strong vaccine, HIV drug sales”. BioPharma Dive (May 1, 2024). Available at: https://www.biopharmadive.com/news/gsk-vaccine-arexvy-shingrix-forecasts/714818/.
- Pfizer Investor Insights “Pfizer Reports Third Quarter Results” (October 31, 2023). Available at: https://insights.pfizer.com/q323earnings/.
- ImmunityBio press release “ImmunityBio Announces FDA Approval of ANKTIVA®, First-in-Class IL-15 Receptor Agonist for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer” (April 22, 2024). Available at: https://immunitybio.com/immunitybio-announces-fda-approval-of-anktiva-first-in-class-il-15-receptor-agonist-for-bcg-unresponsive-non-muscle-invasive-bladder-cancer/
- ImmunityBio press release “ImmunityBio Announces Positive Overall Survival Results of Anktiva Combined With Checkpoint Inhibitors in Non-Small Cell Lung Cancer; Meeting Scheduled with FDA to Discuss Registration Path for ANKTIVA in Lung Cancer.” (April 24, 2024). Available at: https://immunitybio.com/immunitybio-announces-positive-overall-survival-results-of-anktiva-combined-with-checkpoint-inhibitors-in-non-small-cell-lung-cancer-meeting-scheduled-with-fda-to-discuss-registration-path-for-anktiv/): 2047-2064.

If you are interested in our services regarding the development and regulatory aspects of biologics, please don’t hesitate to contact us. Mabion’s specialist offer individualized approach tailored to the biologic and clinical characteristics of the developed drug. Our experience with end-to-end development and manufacturing of biologics gives us an overwhelming advantage over other CDMOs as we perfectly understand the client’s perspective. Click here to obtain more information on our services.
Related resources

Biologics Characterization for Ensuring Product Quality and Consistency
Analytics, Biologics
Monoclonal Antibody Prophylaxis for Infectious Diseases – Passive Immunization and Prevention
Monoclonal antibody, Vaccines

End-to-End Manufacturing of Biosimilars – From Cell Line to Commercial Product
Biosimilars, Manufacturing