Huge spike in the use of GLP-1 analogs amid severe shortages
- The use of GLP-1 analogs (also known as GLP-1 receptor agonists or incretin mimetics) is growing rapidly, with increasing share of users having no diabetes. A recently published analysis reveals that the annual rate of prescriptions in the US surged from 0.22% between 2011 and 2014 to 2.11% in between 2019 and 2023. Meanwhile, the proportion of newly treated patients without diabetes rose to nearly 30% in 2023.
- This growth is mostly attributed to the recent approval of two GLP-1 analogs, semaglutide (Ozempic or Wegovy) and liraglutide (Victoza), for weight management in obese and overweight patients.
- The climbing demand for these drugs driven by expanded indications and increasing off-label use for cosmetic weight loss, led to insurmountable shortages on the market. Manufacturers and regulatory authorities cooperate closely to address this crisis.
Over one-quarter of new GLP-1 receptor agonist users in 2023 had no diabetes, according to a new study of US prescription trends published in Annals of Internal Medicine. In 2019, nearly 90% of patients initiating therapy were diabetics, but by 2023, their share had dropped to just over 70%. Meanwhile, the overall number of patients treated with GLP-1R agonists increased sharply, with the mean annual incidence of new prescriptions rising from 0.22% during 2011-2014 to 0.60% during 2015-2018, and then further to 2.11% in the 2019-23 period.
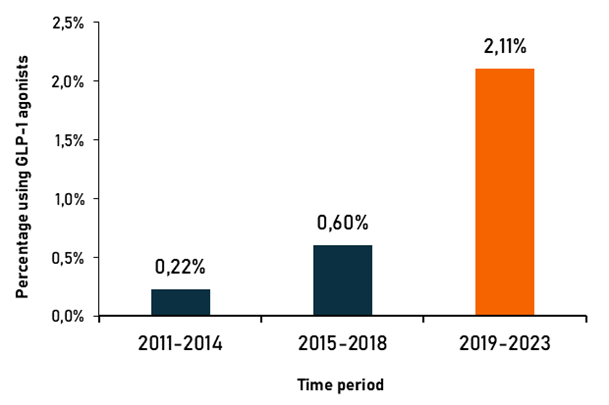
This rapid increase in prescription rates followed the recent approval of two GLP-1R agonists, semaglutide (Ozempic, Wegovy) and liraglutide (Victoza), for weight management in obese or overweight patients. As other members of this medication class, they were originally developed as glucose-lowering agents for type 2 diabetes. However, upon observing substantial weight loss in clinical trials, manufacturers sensed a huge business opportunity and decided to repurpose these drugs for the treatment of obesity – a modern pandemic affecting most of the developed countries.
The GLP-1R agonists quickly became popular as miraculous weight loss medications, which allow to lose even 10-15% of body weight without sacrificing favorite foods or spending hours in the gym. Consequently, the number of GLP-1 receptor agonist users skyrocketed, with an increasing proportion being treated for non-diabetic indications. Predictably, these drugs also started to be used off-label, for cosmetic weight loss, by people seeking to lose a few kilos without having to do strenuous exercises or going through restrictive diets. Although the proportion of such users is still tiny (less than 0.4%), the upward trend is clearly visible in the prescription data. However it should be noted that the overall and off-label use of GLP-1R agonists in 2023 are likely underestimated in the published study, as it doesn’t include the recently approved member of this group, tirzepatide (Mounjaro), which induces even greater weight loss than Ozempic.
A massive surge in demand for GLP-1R agonists driven by new indications and off-label use has led to widespread shortages on the market. Manufacturing capacity constraints have exacerbated the situation, and as of mid-2024 still continue to affect Victoza and Ozempic production. The shortages are particularly dangerous for patients with severe uncontrolled diabetes who rely on these drugs to manage their blood sugar levels. The situation is further complicated by the circulation of counterfeit products, which may contain incorrect doses or hazardous contaminants, posing serious risks to patients.
Multiple solutions have been attempted to resolve the Ozempic crisis, but so far they met only with limited success. Manufacturers have invested heavily in new peptide production facilities and outsourcing the manufacture to various CDMOs. However, this approach has been hampered by the recent COVID-19 pandemic, which strained CDMOs’ capacities and caused severe shortages of equipment and raw materials. The issue has been also tackled by regulatory agencies. EMA has just published recommendations to prioritize the distribution of GLP-1R agonists to high-risk patients and launch awareness campaigns to prevent off-label prescribing. The agency is closely cooperating with market authorization holders and governments to control the crisis.
Unfortunately, these efforts are being thwarted by the new guidelines, encouraging broader use of incretin mimetics in overweight patients, particularly those at high cardiovascular risk. These recommendations were motivated by the results of a recent cardiovascular outcomes trial (SELECT study), performed in obese subjects without diabetes, which reported a 20% reduction in the composite endpoint of cardiovascular death, non-fatal myocardial infraction and non-fatal stroke. Many physicians believe that GLP-1R agonists are underutilized and should be expanded to populations without diabetes or obesity. The GLP-1 receptor agonists seem to be the victims of their own success. Shortages are predicted to continue at least until the end of 2024.
Prepared by:
Sources and further reading
- Yeo, Yee Hui, et al. “Shifting trends in the indication of glucagon-like peptide-1 receptor agonist prescriptions: a nationwide analysis.” Annals of Internal Medicine (2024).
- European Medicines Agency (EMA). EU actions to tackle shortages of GLP-1 receptor agonists (June 26, 2024). Link: https://www.ema.europa.eu/en/news/eu-actions-tackle-shortages-glp-1-receptor-agonists.
- World Health Organization (WHO). Shortages impacting access to glucagon-like peptide 1 receptor agonist products; increasing the potential for falsified versions (Jan 29, 2024). Link: https://www.who.int/news/item/29-01-2024-shortages-impacting-access-to-glucagon-like-peptide-1-receptor-agonist-products–increasing-the-potential-for-falsified-versions.
- Bailey, Clifford J. “Why are GLP-1 receptor agonists in short supply?” British Journal of Diabetes (2022); 22:72.
- Lincoff, A. Michael, et al. “Semaglutide and cardiovascular outcomes in obesity without diabetes.” New England Journal of Medicine 389.24 (2023): 2221-2232.
- Mahase, Elisabeth. “GLP-1 shortages will not resolve this year, EMA warns, amid concern over off-label use.” (2024).