Are mucosal vaccines the future of COVID-19 prevention?
- Researchers are developing mucosal vaccines against COVID-19 that are administered intranasally or via inhalation, enhancing the immunity at the primary site of SARS-CoV-2 infection.
- A newly published study demonstrated that one of such vaccines, bivalent and adenovirus-based, significantly increased IgA antibodies in upper and lower respiratory tracts, and induced durable protection against challenge with SARS-CoV-2 XBB.1.16 virus in non-human primates.
- Mucosal vaccines are expected to reduce viral transmission, and provide broader and more sustained protection against the newly emerging variants than the currently used mRNA vaccines. However, in order to keep their promise, they will have to overcome numerous challenges associated with clinical development, regulatory approval and public acceptance.
The COVID-19 pandemic has highlighted the critical need for effective vaccines, not only to protect against severe disease but also to prevent transmission of the virus. While current mRNA vaccines, such as Comirnaty developed by Pfizer-BioNTech and Spikevax developed by Moderna, have proven to be highly effective at reducing severe illness and death, they have shown limited efficacy in preventing infection and transmission, especially with the emergence of new, highly transmissible Omicron variants like XBB.1.16.
To address these challenges, researchers are exploring new vaccine strategies that could provide better immunity at the primary site of infection: the respiratory mucosa. Direct intranasal administration should stimulate both local and systemic defense mechanisms, affording a more complete protection than the currently used mRNA vaccines. A recent study published in Nature Immunology titled “Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates” provides promising evidence on the feasibility of this approach.
The new study investigated the effectiveness of ChAd-SARS-CoV-2-S, a bivalent, replication-incompetent, adenovirus-based vaccine, in nonhuman primates, with a focus on its ability to elicit mucosal immunity and prevent infection with the XBB.1.16 variant. Twenty rhesus macaques were first immunized with standard Wuhan-strain mRNA vaccine (Spikevax) and then, seven months later, randomized into 3 treatment groups. Two groups received investigational ChAd-SARS-CoV-2-S vaccines containing Wuhan and BA.5 strain spike proteins via nebulizer or atomizer, while a single control group received bivalent mRNA vaccine encoding the same two strains. The control mRNA-boosted monkeys served as a benchmark for comparing immune responses and efficacy.
The researchers found that a booster dose of the mucosal adenovirus vaccine led to a significant increase in IgA antibodies in the nasal mucosa, as well as a durable systemic immune response. Most importantly, the vaccinated animals were protected from infection with XBB.1.16, highlighting the potential of mucosal vaccines to prevent not only severe disease but also transmission. In contrast to ChAd-SARS-CoV-2-S, protection obtained with intramuscularly administered mRNA vaccine was limited only to the lower respiratory tract. No culturable virus could be detected in bronchoalveolar fluids following the SARS-CoV-2 challenge, whereas high amount of the virus was found in nasal cavity, where replication continued until the final day of experiment. Although intranasal boosting caused lower rise in neutralizing antibodies, their titers remained more stable during the 5 months of observation, without substantial waning that is characteristic for intramuscular mRNA vaccines. IgA levels in nasopharyngeal samples spiked after administration of mucosal booster, but remained unaltered or even decreased after the mRNA vaccination. Immunization with mucosal preparation was also found to significantly expand S-specific cellular immunity, suggesting long-term protection against the most severe consequences of SARS-CoV-2 infection. Similar findings have been previously reported for other intranasal or aerosolized vaccine candidates, including those incorporating live attenuated viruses or adjuvanted proteins.
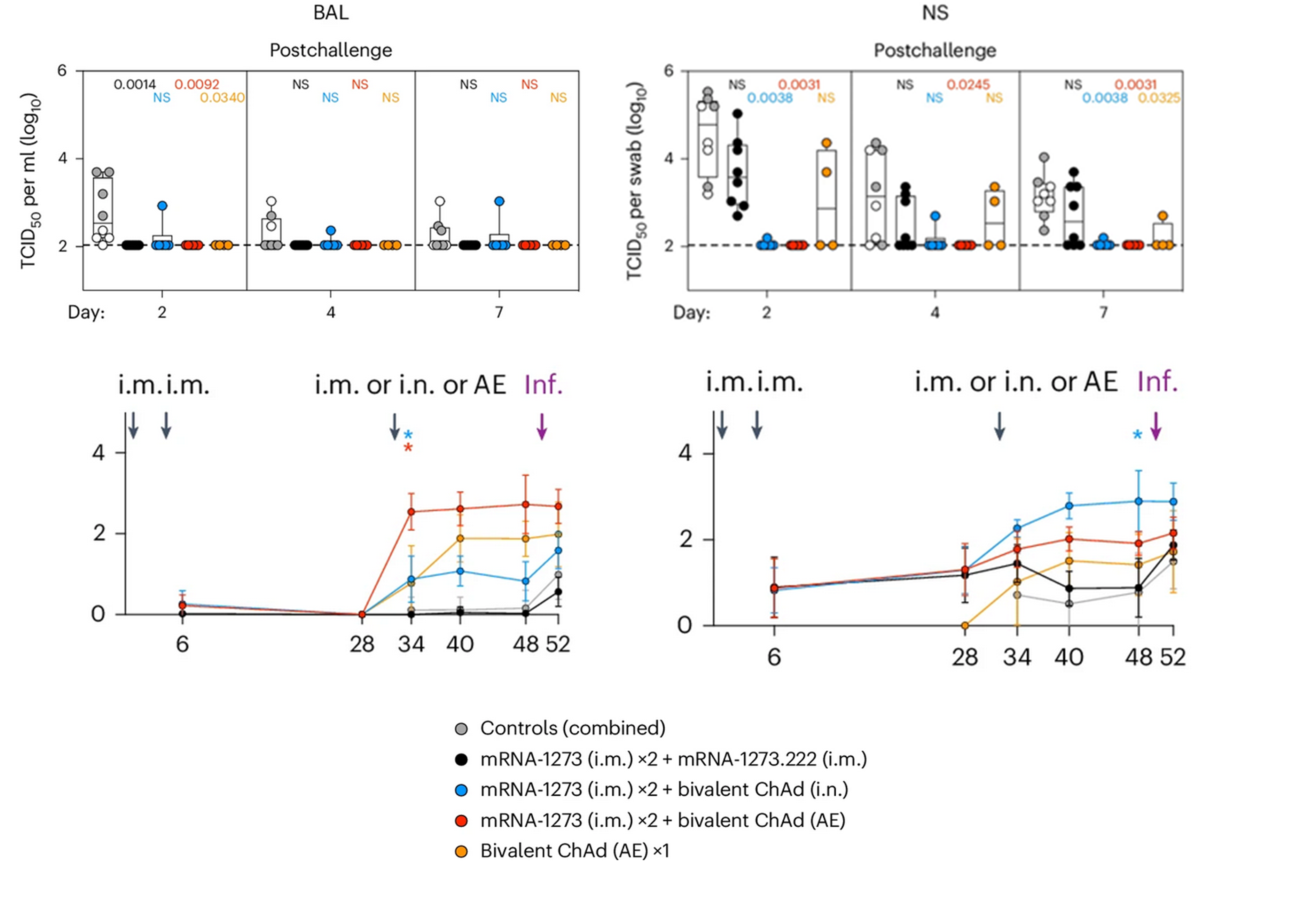
Upper graphs: Viral replication after SARS-CoV-2 XBB.1.16 challenge in rhesus monkeys vaccinated with 2 x monovalent mRNA + bivalent mRNA, 2 x monovalent mRNA + bivalent ChAd (intranasal), 2 x monovalent mRNA + bivalent ChAd (aerosolized) or bivalent ChAd (aerosolized) alone in bronchoaveloar fluids (left graph) and nasopharyngeal fluids (right graph).
Lower graphs: IgA titers in the four study groups following the primary vaccination, booster dose and viral challenge.
Mucosal vaccines could offer several important advantages over the standard mRNA vaccines. First of all, they would not only afford individual protection, but also reduce viral shedding and prevent person-to-person transmission. Prior to the emergence of Omicron variant, Comirnaty and Spikevax could also limit virus spread, but unfortunately they are now unable to do so, even with the availability of adapted preparations and administration of additional boosters. Secondly, intranasal vaccines are expected to provide broader and more durable protection. By inducing robust mucosal immunity via IgA production stimulation they could become powerful weapons against the rapidly evolving variants, eliminating the need for annual revaccinations and frequent composition updates. It is also possible that vaccines delivered directly to the infection site will require lower antigen doses to achieve the desired response, thus substantially lowering the manufacturing costs.
Despite these encouraging data, commercialization of mucosal COVID vaccines is still a long way off. Large-scale clinical studies have to be conducted and numerous regulatory hurdles dealt with before the new generation of vaccines could replace or at least complement the currently employed mRNA and protein-based products. Confirming non-inferiority to the available vaccines should not be problematic, but demonstrating the ability to provide sustained immune response and confer broad protection against the newly emergent variants would be an enormous challenge, requiring clever development strategy as well as large amount of financial and human resources. As with any new technology, public perception and acceptance will play a significant role in the success of mucosal vaccines. Education and communication efforts will be necessary to build trust and address potential concerns about safety, efficacy, and the novel route of administration, to prevent another backlash against vaccines similar to the one with mRNA technology. However, if successfully developed and deployed, mucosal vaccines could play a pivotal role in controlling the impact of future COVID-19 waves.
Prepared by:
Sources and further reading
- Gagne, M., Flynn, B.J., Andrew, S.F. et al. “Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates” Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01951-5.
- McMahan, K., Wegmann, F., Aid, M. et al. “Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques” Nature 626, 385–391 (2024). https://doi.org/10.1038/s41586-023-06951-3.
- Stark, Felicity C., et al. “Intranasal immunization with a proteosome-adjuvanted SARS-CoV-2 spike protein-based vaccine is immunogenic and efficacious in mice and hamsters.” Scientific Reports 12.1 (2022): 9772.