Zolbetuximab demonstrates significant benefit in gastric cancer and receives approval in the EU
- Zolbetuximab, a monoclonal antibody targeting CLDN18.2, demonstrated significant benefits in advanced gastric and gastroesophageal junction cancer. The SPOTLIGHT and GLOW trials reported improvement both in progression-free and overall survival, over chemotherapy alone.
- Zolbetuximab reduced the risk of death by 25%, with OS extended to 18.23 months in the SPOTLIGHT trial and 14.39 months in the GLOW trial. These improvements were achieved with manageable side effects, predominantly nausea and vomiting.
- The recent approval of zolbetuximab by the EMA marks a significant advance in precision oncology, validating CLDN18.2 as a therapeutic target.
Zolbetuximab, a first-in-class monoclonal antibody targeting claudin 18.2 (CLDN18.2), has shown remarkable improvements in both progression-free survival (PFS) and overall survival (OS) in patients with advanced CLDN18.2-expressing gastric and gastroesophageal junction cancer. This breakthrough was confirmed in two pivotal Phase 3 trials, SPOTLIGHT and GLOW, culminating in the recent approval of zolbetuximab by the European Medicines Agency (EMA).
The SPOTLIGHT trial demonstrated that combining zolbetuximab with mFOLFOX6 chemotherapy resulted in a median PFS of 10.61 months compared to 8.67 months for chemotherapy alone. At first glance, delaying disease progression by 2 months may seem like a small benefit, but it is quite significant considering that the treated patients had advanced and unresectable or metastatic cancer, for which it is extremely difficult to achieve any improvement. Similarly to PFS, median OS was extended to 18.23 months with zolbetuximab versus 15.54 months with placebo, showing a 25% reduction in the risk of death. The GLOW trial, which employed a different chemotherapeutic regimen (CAPOX), mirrored these findings, with zolbetuximab group achieving a median PFS of 8.21 months compared to 6.80 months in the control group, and OS extended to 14.39 months from 12.16 months. Efficacy of the novel drug was further highlighted by high objective response rates (ORR) of up to 60.7%, alongside manageable side effects such as nausea and vomiting, which were more frequent but consistent with expectations for this population.
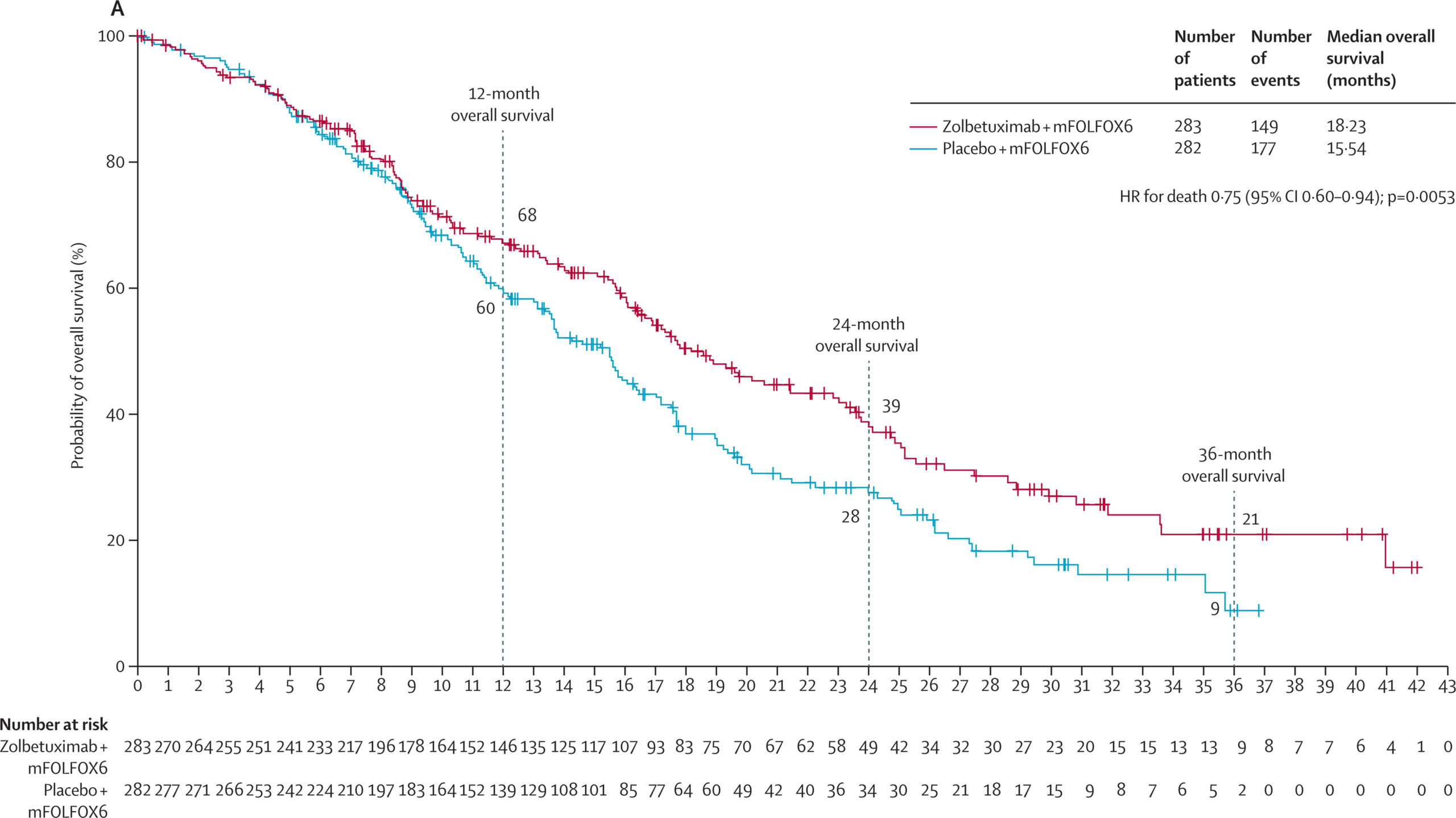
Overall survival in zolbetuximab versus placebo group in the SPOTLIGHT trial (Source: Shitara et al. 2023)
The two completed trials clearly validate CLDN18.2 as a therapeutic target for gastric cancers. CLDN18.2 is a member of the claudin family of proteins, which are critical components of tight junctions in epithelial cells. In normal tissues, CLDN18.2 is mainly expressed in the stomach lining, but in cancer cells, its expression can expand, causing accelerated tumor growth. Importantly, this protein is absent or minimally expressed in most other healthy tissues, making it a safe target for cancer therapies. Zolbetuximab, a monoclonal antibody, is designed to bind specifically to CLDN18.2 on the surface of cancer cells, leading to the activation of immune responses that kill these cells. The targeting of CLDN18.2 represents a novel approach in precision oncology, offering a highly specific treatment option with reduced damage to normal tissues.
The EMA’s approval of zolbetuximab represents another step forward in precision oncology, providing a new, highly effective option for patients with advanced gastric cancers – a population historically underserved by available treatments. Gastric cancer, also known as stomach cancer, is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths. It often presents at an advanced stage due to its non-specific symptoms, which may include indigestion, stomach pain, nausea, and weight loss. Because of late diagnoses, the overall 5-year survival rate remains low, particularly in metastatic and unresectable cases, where standard chemotherapy offers little benefit. Zolbetuximab’s approval marks a turning point, offering hope for improved survival and quality of life for those affected by this aggressive cancer.
Prepared by:

Adam Tuszyner
Registration Dossier Specialist, Pharmacovigilance Specialist
Sources and further reading
- Shitara, Kohei, et al. “Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma.” New England Journal of Medicine (2024).
- Shitara, Kohei, et al. “Zolbetuximab plus mFOLFOX6 in patients with CLDN18. 2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial.” The Lancet 401.10389 (2023): 1655-1668.
- Wahner, Ashling. “Zolbetuximab Plus Chemotherapy Wins EU Approval for Advanced CLDN18.2+ Gastric/GEJ Cancer” OncLive (September 20, 2024). Link: https://www.onclive.com/view/zolbetuximab-plus-chemotherapy-wins-eu-approval-for-advanced-cldn18-2-gastric-gej-cancer.
- Shitara, Kohei “Zolbetuximab Improves Survival in Gastric Cancer, New Phase 3 Data Confirm” Oncology New Central (June 17, 2024). Link: https://www.oncologynewscentral.com/video/zolbetuximab-improves-survival-in-gastric-cancer-new-phase-3-data-confirm.