Gene to Vial: End-to-End Development
At Mabion, we are proud to offer a full set of drug development services for biologics. We contribution our customers all the way with gene to vial biologics support to first-in-human trials.
Our integrated end-to-end approach allows for seamless coordination across all phases, from the initial gene sequence to the final vialed product, ensuring efficiency, quality, and regulatory compliance. Our flexible offering allows clients to choose a full-package solution, encompassing every aspect of biologic drug development, or select bespoke services that best suit their unique project requirements. Utilizing our deep expertise in biologics development and manufacturing, Mabion is committed to accelerating innovative therapies, reducing time to market, and ensuring the highest quality standards.
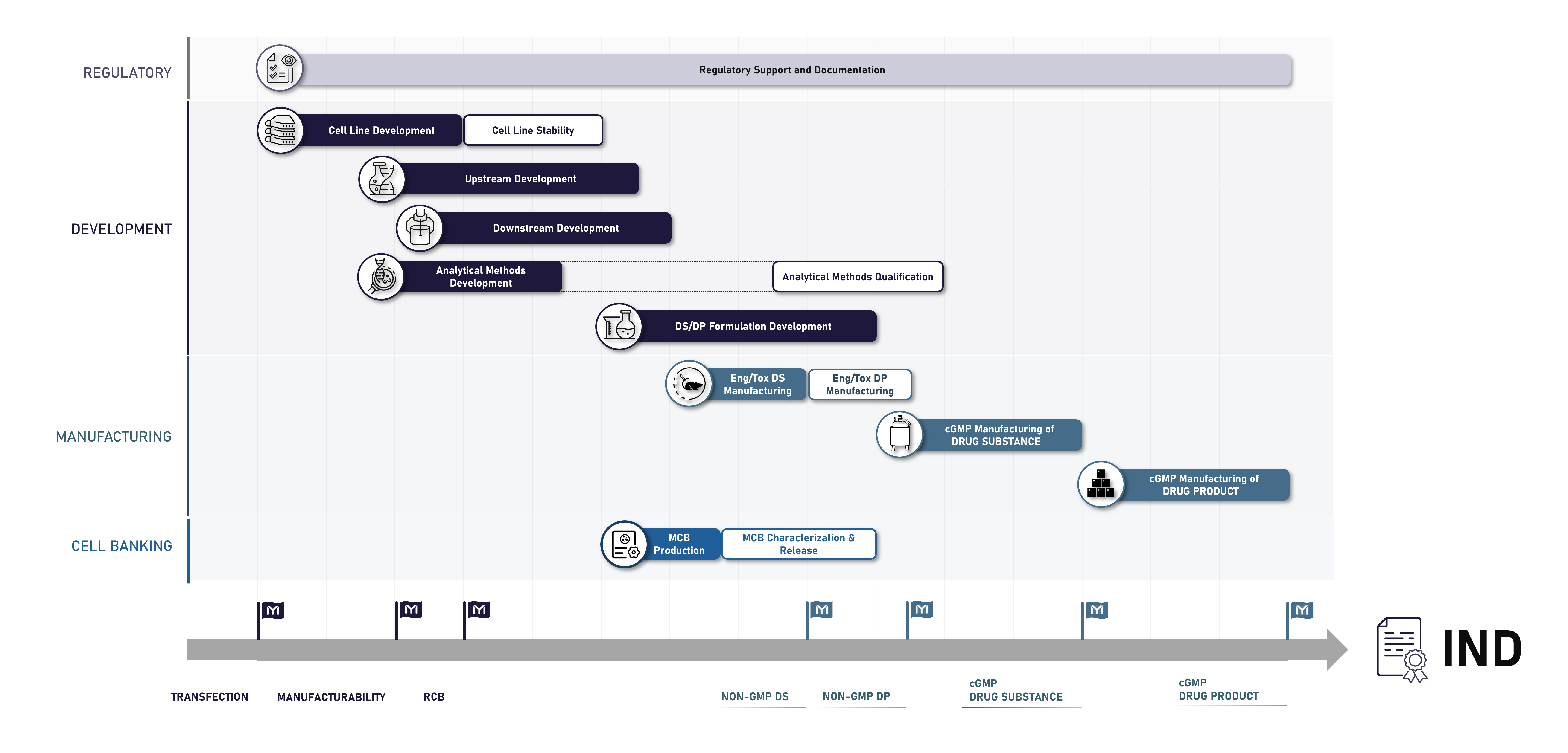
The figure below represents Mabion’s drug development pathway starting from cell line development and ending with successful cGMP manufacturing of the drug product, ready for first-in-human studies.
All Mabion’s services are provided with full regulatory support, which guarantees compliance with all applicable EU and US regulations. Our specialists’ oversight provides you confidence that your marketing authorization application and drug documentation meets the highest international standards.
Cell line development and cell banking
- Cell Line Development
- Non-GMP Research Cell Banks (RCBs)
- cGMP Master Cell Banks (MCBs) and Working Cell Banks (WCBs)
The foundation of any successful biologic product lies in a robust and high-performing producer cell line. Mabion offers end-to-end services for generating stable, high-producing cell lines that express your target protein with the right attributes. Our comprehensive approach ensures the development of a cell line that meets your specific product goals.
Once the optimal cell line is established, we offer cell banking services to preserve your valuable cell lines for future use. Our fully cGMP-compliant cell banking services include the creation of master cell banks (MCB) and working cell banks (WCB), ensuring long-term stability and security of your production cell lines. All cell banks are stored under controlled conditions, and each is rigorously characterized and tested for genetic stability, sterility, Mycoplasma contamination, and viability to meet regulatory standards.
Learn more about our Cell Line Development and cell banking services.
Drug Substance manufacturing
- Biosimilars
- Innovative antibodies (incl. multispecific)
- Other therapeutic proteins
- Vaccine antigens
- Analytical and QC support
Mabion’s Drug Substance Manufacturing services are built on a foundation of quality, efficiency, and scalability. We specialize in the production of drug substances, whether for early clinical development, large-scale commercial production, or anything in between. Our state-of-the-art facilities are designed to meet the stringent requirements for biologics manufacturing, including full cGMP compliance and the highest standards of sterility and safety.
We provide a comprehensive approach to upstream and downstream manufacturing. In upstream processes, we use high-performance cell culture technologies to maximize the yield of your biologic product while ensuring consistency and robustness. Our downstream processing expertise focuses on advanced purification methods that deliver high-quality drug substances with optimal purity, identity, and potency. Mabion’s facilities are equipped to handle a range of production scales, from small clinical batches to large-scale commercial manufacturing, ensuring that your product can be efficiently scaled-up as needed.
Mabion’s quality control and in-process testing capabilities ensure that every batch of drug substance meets or exceeds regulatory requirements. Our advanced analytical methods provide critical data on product quality throughout the manufacturing process, allowing for the timely detection and resolution of any issues. With a focus on delivering high-quality drug substances in a timely and cost-effective manner, Mabion is your partner in bringing life-saving biologic therapies to market.
Learn more about our Drug Substance manufacturing services.
Process development
- Process development for Phase 1
- Process scale-up
- Process optimization
- Process characterization
Our process development services are designed to create scalable and robust manufacturing processes that consistently deliver high-quality biologics. Mabion’s process development expertise spans both upstream and downstream processes, ensuring that we can optimize every aspect of biologic production.
In upstream development, we focus on optimizing cell culture conditions, media formulations, and feeding strategies to maximize product yield, quality, and consistency. We design processes that can be scaled smoothly from small-scale development to large-scale commercial production. In downstream development, we specialize in purification strategies tailored to the specific characteristics of your product, ensuring high purity and efficient recovery.
Our process development services also include process characterization and process validation to meet regulatory expectations for commercial manufacturing. Whether you’re in the early stages of clinical development or preparing for commercial scale-up, Mabion’s process development team ensures that your biologic product is produced efficiently, consistently, and cost-effectively.
Learn more about our Process development services.
Analytics
- Methods optimization, qualification and validation
- Drug characterization
- Routine and in-process testing
- GMP release testing
- GMP stability testing
Mabion provides comprehensive analytical method development services to ensure accurate, reliable, and reproducible characterization of your biologic product. We offer a full range of analytical capabilities designed to assess critical quality attributes (“CQAs”) such as potency, purity, identity, and stability. Our method development services are aligned with regulatory expectations, ensuring that all methods are suitable for both clinical and commercial use.
Our services include the optimization and validation of analytical methods, ensuring they are robust and fit-for-purpose throughout the product lifecycle. In addition to developing new assays tailored to your product’s unique characteristics, we can adapt and validate existing methods to meet regulatory standards.
Mabion also offers comparability and biosimilarity studies for biosimilars, as well as reference standard characterization and impurities profiling. Our advanced analytical technologies ensure that every aspect of your biologic product’s quality is monitored and controlled.
Our analytics team works closely with process development and manufacturing to support real-time product release, stability studies, and comparability testing, ensuring that your product maintains its CQAs throughout its lifecycle.
Analytical services performed at our facility include comprehensive testing for potency, purity, identity, and impurity profiles using a wide array of techniques such as ELISA, HPLC, SDS-PAGE, and mass spectrometry. We also offer in-process testing to monitor critical parameters at each stage of production, ensuring early detection of potential issues and maximizing process efficiency.
For clients transitioning from development to manufacturing, we specialize in method transfer and adaptation to ensure smooth transition into production while meeting all regulatory and cGMP requirements. Mabion also ensures rigorous cGMP quality control testing throughout every phase of the manufacturing process, with services such as release testing, stability testing, biosafety testing (sterility, endotoxin, Mycoplasma), and raw material testing.
Learn more about our Analytical services.
Fill & Finish
- Our equipment
- Sterilization
- Filling
- Packaging and serialization
- Product inspection
- Full in-process and release testing
The final step in the gene to vial biologics manufacturing process is critical to ensuring the drug product’s sterility, stability, and integrity. Mabion’s Fill & Finish services offer clients fully cGMP-compliant solutions for vialing drug substances, whether for clinical trials or commercial production. We provide aseptic filling capabilities in a sterile environment, minimizing contamination risks and ensuring product safety.
Our Fill & Finish services include final drug product formulation, sterile filtration, and precise filling into vials. We also offer visual inspection and packaging services ensuring the final product is ready for distribution. Throughout the Fill & Finish process, our experienced team ensures strict adherence to regulatory standards, including thorough documentation and quality control checks at each stage.
Learn more about our Fill & Finish services.