A synergy of excellence: Novavax-Mabion partnership on vaccine during unprecedented times amid the COVID-19 pandemic
Mabion, Vaccines

- Starting in late 2021, Mabion supported Novavax in their endeavor by performing COVID vaccine manufacturing and analytical services. High quality and timely performance enabled the extension of the cooperation to include more QC activities, such as those related to its updated XBB.1.5 strain vaccine.
- Mabion as a reliable partner for outsourcing the manufacturing of biologic products became part of Novavax’s service providers network as well as their mission to develop effective and safe vaccines.
- The COVID-19 pandemic caused an unprecedented crisis that had to be quickly addressed by the development of effective vaccines. Novavax was among several companies that took up the gauntlet and managed to introduce a vaccine (Nuvaxovid) in less than two years since the beginning of the pandemic.
COVID-19 pandemic – the fear and the hope
Imagine you are back in Spring 2020. What do you see? A novel coronavirus is spreading out of control around the world causing a pandemic outbreak of unknown causes and unimaginable consequences. The world has literally locked down. The lives of millions of people are at risk. Global supply chains are broken, and disinfectants, antiviral agents, or personal protection equipment such as face masks are virtually unavailable. Millions of enterprises must shut down, which soon led to the worst global recession since World War II1,2.
The scientific community responds immediately. It is commonly understood that the raging pandemic can be overcome only if the human population is vaccinated and reaches what is known as “herd immunity.” The world’s greatest academic and research institutes along with the pharmaceutical industry start cooperating to develop affordable, easy-to-produce, and highly effective COVID-19 vaccines. The first achievements are reported in record time. Novavax, one of the very few companies that succeed in formulating an innovative protein-based adjuvanted vaccine candidate, starts scale-up and clinical trials.2 However, this complex endeavor that typically takes a decade or more to achieve, encounters challenges. To manufacture a sterile medicinal product for human use, stringent regulatory requirements and high-quality standards must be fulfilled. It is no longer possible to stay in a research laboratory.3
The capabilities of existing plants are limited, whereas building new ones is not an option under current circumstances. Fast scale-up and technology transfer themselves pose another extremely complex, inevitable challenge. This is the turning point in further drug development and commercialization. Cooperation with CDMOs will play a crucial role. This is the point when Mabion and Novavax meet and initiate discussions4,5 . Soon after this event, an adventurous, long-term, and rewarding partnership will be established. But let’s start from the beginning.
Let’s organize, let’s agile
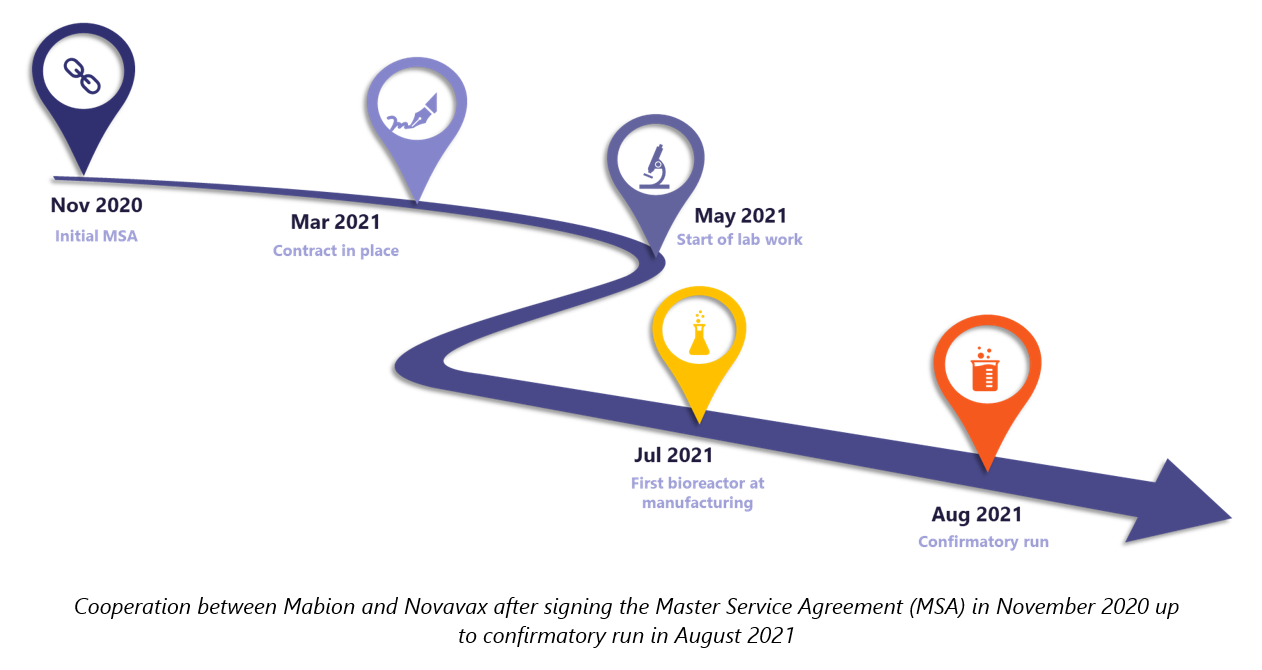
Officially, the cooperation between Novavax and Mabion started on March 3rd 2021, the day when the COVID-19 vaccine Master Service Agreement was signed.4,5 Yet, details regarding the scope of the contract, CAPEX, OPEX, and production plans have been under development since November 2020 when Mabion and Novavax representatives connected for the first time. As the agreement came into force, the project team was instantly assigned, the kick-off meeting was scheduled and the operational work began.
The Master Service Agreement detailed the technology transfer from Novavax to Mabion at a scale enabling commercial manufacturing of the drug substance. The contract was divided into seven work packages – some of them organized sequentially, with others to be implemented in parallel. Each work package was managed by a leader responsible not only for the progress but also for compliance with the outcomes of other work packages. Teams consisted of subject matter experts, specialists, and operators from various functions. Necessary documents were shared online via a secured cloud-based system. The status of day-to-day progress was constantly available to Novavax from the very first day, while traceability of changes and records was ensured.
As COVID-19 continued in full force, companies were rushing to apply for approvals for new products that would foster the global vaccination process and ideally reverse the course of the pandemic. Everyday work in such a dynamic environment was extremely challenging, in large part because the required workload was disproportionately greater than the available resources. To ensure productive communication, Novavax and Mabion set regular video-conference slots, including JOC (Joint Operating Committee) and JSC (Client-Sponsor Senior Management Steering Committee) meetings together with periodic subject-related calls (logistics, QC, QA, or manufacturing).
The issue of time zone difference was easily solved by adapting work schedules and adjusting the available resources. Another arduous undertaking lay in organizing raw materials that were necessary for production. Global supply chains were then broken, which meant deliveries were significantly delayed, while raw materials and consumables were often entirely out of stock. Mabion and Novavax swiftly dealt with the situation. There were a lot of efforts to map availability, to plan precisely, and to be efficient in negotiations. All of that paid off and the needed raw materials were successfully secured. What is also important, the arrangement also guaranteed stocks for future commercial manufacturing.6
“The exceptional capabilities, state-of-the-art technologies and commitment to quality make Mabion a highly valuable partner for any company wishing to outsource or augment key manufacturing processes”
John Kutney, Vice President, Manufacturing, Novavax7
Mid 2021: Economies are still in a downturn and many societies are in desperate need to obtain a sufficient number of vaccines. Every day counts. Pharma companies cope with the situation differently, but only a few can report significant success. The feasibility phase at Mabion, during which the process was transferred and fully scaled up, took around one quarter, meaning the preliminary goals were achieved far ahead of schedule.
Furthermore, Mabion was able to perform a successful scale-up to the final commercial volume in the first bioreactor run, and subsequent runs only confirmed the preliminary, favorable results. The project of technology transfer, starting from the day of signing the contract to the day of final report delivery, took only 30 weeks – an extraordinary achievement considering the challenging pandemic conditions.
Following the initial fruitful cooperation and promising results, Novavax opened negotiations for full-scale commercial manufacturing. The scope of the contract was gradually expanded by signing further SOW’s, both in the area of GMP manufacturing and developmental projects.
The daring beginning – feasibility studies
A well-designed and performed technology transfer shall be preceded by a thorough fit assessment that ensures the choice of the best adaptations. That was also the case for the Mabion-Novavax project. The project started from the laboratory scale runs at a scaled-down model platform which was followed up by the scale-up to the final bioreactor scale for manufacturing. Such an approach delivered reliable data in the most cost-effective and timely manner. The platform developed by Mabion for monoclonal antibodies was adopted for the project and worked perfectly for the manufacturing process of Novavax vaccine antigen, which consists of the expression of SARS-CoV-2 rS protein in a baculovirus-transfected insect cell line.

Predictable and easy-to-control parameters of orbital shaking bioreactors resulted in a repeatable process. All acceptance criteria were met, and the cell culture profiles were comparable, achieving the desired yield of protein. Simultaneously to the process transfer, the suitability of analytical methods was checked. With feasibility studies completed and analytical methods transferred, Mabion was prepared to initiate GMP manufacturing.
Mabion GMP manufacturing of COVID vaccine and logistics
Prompt technology transfer, finalized with a successful SARS-CoV-2 rS drug substance process validation (for Wuhan variant) made the Mabion site ready for commercial manufacturing.
Thanks to constructive teamwork and a well-defined training plan, operators quickly acquired knowledge on this new process. The process was validated; drug substance manufactured by Mabion met all specification criteria.
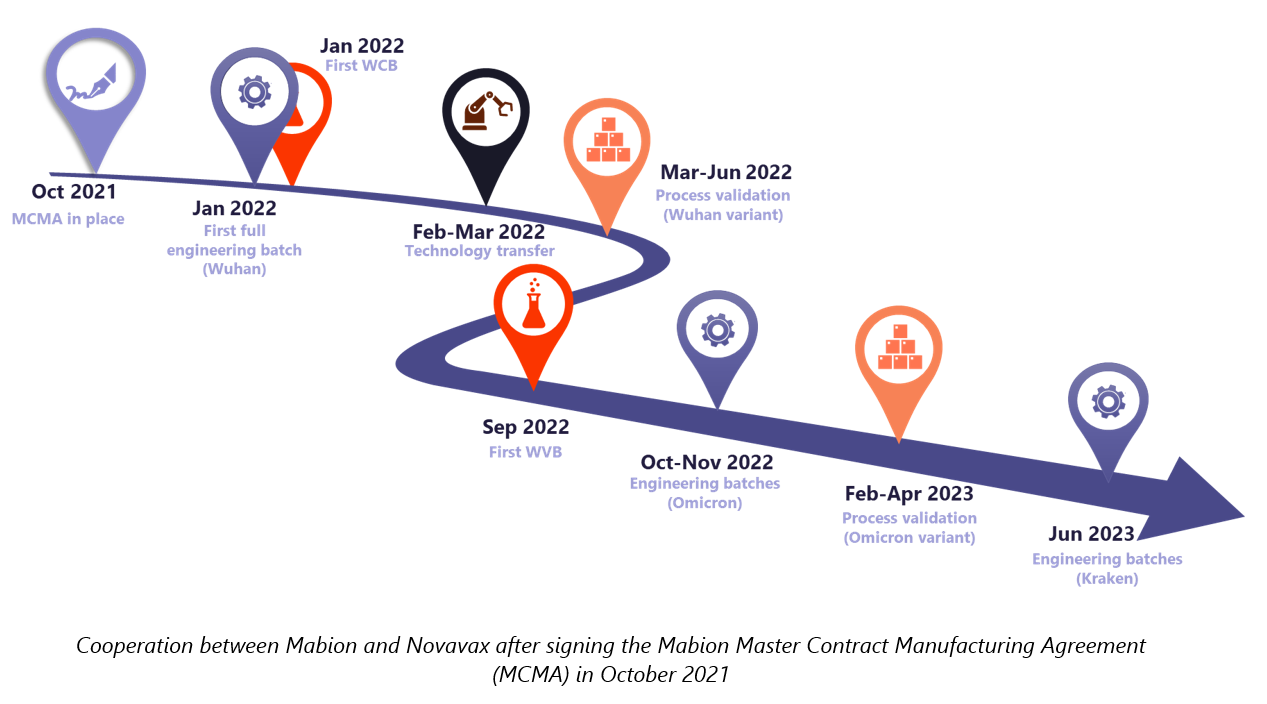
With the next variants of SARS-CoV-2 emerging and shaping the course of the pandemic, there appeared an urgent need to adapt the vaccine. Novavax requested for Mabion to perform a set of technological trials dedicated to the manufacturing of the Omicron variant of the SARS-CoV-2 rS drug substance and extended the agreement.10 Similarly to the Wuhan case, both technology transfer and process validation were successfully completed in the first attempt. Mabion also had a chance to support Novavax in a third project which was focused on technical runs for the XBB.1.5 variant.11
In the meantime, it turned out that Novavax needed to develop new batches of GMP working cell banks. Mabion had the necessary facilities and equipment required for this task. The efforts of both teams allowed for the effective transfer and completion of the outsourced task.12 Mabion in turn – once again, was capable of delivering a satisfactory product. The best proof of this was the next working order for working virus cell stocks.13
Logistics was another important area of cooperation, as Mabion owns a dedicated car fleet and GMP-compliant storage and transportation services at controlled conditions. Novavax found those capabilities valuable and signed a new statement of work for logistics and storage services.14
Finally: during the process validation, several associated studies were commissioned, including hold time, stability, and resin lifetime studies.15-17 All in the GMP standard, all accompanied by thorough analytical testing, and all vital for regulatory purposes. By collaborating on the development of the COVID-19 vaccine, Mabion has contributed to the fight against a global pandemic.
Quality is our second name – Quality control
Analytics of proteins is a complex and multidimensional aspect of drug manufacturing. It comprises physicochemical methods, biological assays, and microbiological tests. Moreover, protein-based medicinal products are intended to be sterile, thus advanced environmental monitoring plays an indispensable role. Having developed a protein-dedicated panel in 2020, Mabion has been already capable of offering all aforementioned analytical methods to its clients, including but not limited to in-process testing, release testing, and stability studies. In reference to the SARS-CoV-2 rS project, Novavax initially requested the transfer of analytical methods, which were necessary for the control of product manufacturing.5
However, as the cooperation between the companies continued, Novavax expanded the provided services. In January 2022 Novavax decided to further extend the scope of partnership and include the new QC-related tasks.18 Since that point, the methods were to be used for testing both in-house samples and samples from other Novavax partner sites. During subsequent months, Novavax gradually extended the contract by new orders, first in May 2022 (testing of drug product), then in June 2022 (stability studies for intermediate products and process solutions), August 2022 (stability studies of drug substance), and November 2022 (peptide mapping of drug substance and drug product of in-house and samples from other facilities).15,17,19,20 Peptide mapping in Mabion as an identity test was also used. Additionally, analytics complemented other activities (cell bank preparation, chromatography resins stability).12,16
Striving for best practices – Quality assurance and regulatory support
None of the above CDMO services would be possible without adopting adequate quality measures and controls. Mabion, having an integrated pharmaceutical quality system in place, supervised all Novavax product- and process-related activities, while the degree to which controls were applied was proportional to the project phase. From the beginning, QA was fully involved in risk management, training, and project implementation.5
Hundreds of documents were released at that time, including study plans and protocols, new technological procedures and instructions, reports, and record templates. The list of qualified vendors was extended by new suppliers and new materials were released in QC. Novavax was notified about quality-related events as soon as they were defined. All activities above were covered by a separate work package as part of the MSA. Just before GMP manufacturing was initiated, a separate Quality Agreement came into force.21 Beginning in April 2022, Mabion became an official SARS-CoV-2 rS drug substance manufacturer, enlisted in the National Register of Manufacturers, Importers and Distributors of Active Substances.22
Mabion is a GMP-certified manufacturer of biological sterile injectables, therefore it undergoes regular GMP inspections and was audited by Novavax on an annual basis. One may say that frequent auditing is a sufficient method of oversight. However, Mabion took further steps and agreed to a full-time hired person-in-place. During the first two years of the project, Novavax specialists were present on site, both during the manufacturing process and in day-to-day operations, thanks to which many technological risks could be mitigated early.
In the same way as the QA team is accountable for good manufacturing practices, the regulatory team shares the responsibility of widely defined regulatory compliance. In the Novavax project, the team took the lead in technology transfer design and process validation, supervision over analytical method transfers, their validation, and supplementary studies (e.g. stability studies of resins, drug substance, drug product and its intermediates, hold time studies). The documentation for process validation and analytical methods complied with regulatory standards for the EU/US marketing authorization documentation.
“The history of our partnership with Mabion (…) is a best testimony of the high standards, technical expertise, and cooperation skills among trusted partners”
John Kutney, Vice President, Manufacturing, Novavax7
Excellence leading to success
Despite being a new entity in the CDMO market, Mabion used experience built over the previous 16 years of working on biopharmaceutical products which has led to becoming a successful service provider of the most complex biopharmaceutical modalities. Mabion proved that a dedicated team of talented, well-trained, and committed employees, together with their determination, openness and great flexibility towards the Client, enable delivering services at the highest quality standards.
In less than two years Novavax successfully registered its product with EMA and FDA.23-26 Mabion as a collaborator of this success actively participated in the implementation of Novavax’s global strategy, with Novavax’s Vice President providing his support:
“Throughout more than two years of our collaboration, we were extremely satisfied by their commitment to the undertaken projects and regarded the Mabion team as an integral part of our team, equally committed to our mission to develop effective and safe vaccines to protect health”
John Kutney, Vice President, Manufacturing, Novavax7
Prepared by:
Aleksandra Stasiak
References
- The World Bank. [2020]. COVID-19 to Plunge Global Economy into Worst Recession since World War II. Available at: https://www.worldbank.org/en/news/press-release/2020/06/08/covid-19-to-plunge-global-economy-into-worst-recession-since-world-war-ii.
- Bok K., Sitar S., Graham B.S., Mascola J.R. [2021]. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity 54. Available at: 10.1016/j.immuni.2021.07.017.
- EC. [2022]. Annex 1: Manufacture of Sterile Medicinal Products. The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use.
- Healthcare, Market Experts. [2021]. Mabion to produce a vaccine against COVID-19 Novavax? Available at: https://healthcaremarketexperts.com/en/news/ma-and-investments/mabion-to-produce-a-vaccine-against-covid-19-novavax/.
- Mabion. [2021]. 15/2021. Entering into a master service agreement with the first work order with Novavax Inc. for contract services related to the COVID-19 vaccine program. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2021]. 45/2021. Update on the cooperation with Novavax Inc. on the COVID-19 vaccine program. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Kutney J. [2023]. Recommendation Letter. Novavax.
- Tilles, D. [2021] Covid vaccine antigen to be produced in Poland. Notes from Poland. Available at: https://notesfrompoland.com/2021/10/11/covid-vaccine-to-be-produced-in-poland/.
- Mabion. [2021]. 52/2021. Information on the execution of a commercial manufacturing agreement with Novavax, Inc. for the period of 2022-2025. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 31/2022. Broadening and extension of cooperation with Novavax, Inc. – Omicron variant. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2023]. MABION S.A. Directors’ Report for the first half of 2023. Periodic reports. Available at: https://www.mabion.eu/wp-content/uploads/2023/09/Directors-Report-for-the-1H23_ENG.pdf.
- Mabion. [2022]. 2/2022. Mabion receives an additional order for the production of cellular banks under the manufacturing agreement with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 25/2022. Extension of cooperation with Novavax. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2023]. 4/2023. Extension of cooperation with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 18/2022. Extension of cooperation with Novavax. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 21/2022. Extension of cooperation with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 26/2022. Extension of cooperation with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 3/2022. Mabion receives an additional order to provide analytical product quality control services under manufacturing agreement with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 17/2022. Extension of the scope of services under of manufacturing agreement with Novavax. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 34/2022. Extension of cooperation with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2021]. 63/2021. Mabion enters into a quality agreement as part of its collaboration with Novavax, Inc. Current reports. Available at: https://www.mabion.eu/current-reports/.
- Mabion. [2022]. 11/2022. Registration of the Company as the manufacturer of the SARS-CoV-2 rS active substance in the Register of the Chief Pharmaceutical Inspectorate. Current reports. Available at: https://www.mabion.eu/current-reports/.
- EMA. [2021]. EMA recommends Nuvaxovid for authorization in the EU. News. Available at: https://www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu.
- EMA. [2023]. EMA recommends approval of adapted Nuvaxovid COVID-19 vaccine targeting Omicron XBB.1.5. News. Available at: https://www.ema.europa.eu/en/news/ema-recommends-approval-adapted-nuvaxovid-covid-19-vaccine-targeting-omicron-xbb15.
- FDA. [2022]. Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted. FDA news release. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-emergency-use-novavax-covid-19-vaccine-adjuvanted.
- FDA. [2023] FDA Authorizes Updated Novavax COVID-19 Vaccine Formulated to Better Protect Against Currently Circulating Variants. FDA news release. Available at: https://www.fda.gov/news-events/press-announcements/fda-authorizes-updated-novavax-covid-19-vaccine-formulated-better-protect-against-currently.
Related resources
Time to Market Strategy – 5 CDMO Tactics to Speed Up Biologic Launches
Biologics, Drug development, Manufacturing

Navigating OOX Results: Effective Analysis and Management in CDMO Laboratories
Analytics, Manufacturing, Regulatory

Mammalian cell lines as a platform for protein vaccine production
Cell culture, Drug substance, Manufacturing, Proteins, Vaccines