Bioanalytical assay strategies and considerations for validation of B cells-depletion assay by flow cytometry
Bioanalytics, Biologics
- Pharmacodynamics of B-cell-depleting therapeutics is assessed with a combination of direct and indirect methods, including bioanalytical flow cytometry, which is considered a gold standard for measuring B-cell counts in whole blood.
- Sensitivity is a crucial parameter in bioassays, especially those designed to monitor the pharmacodynamics of cell-depleting therapeutics. Assessing and validating sensitivity involves addressing challenges related to biological variability and lack of biological standards with different levels of dedicated cell populations.
- “Fit-for-purpose” validation of a bioanalytical flow cytometry assay ensures that the assay is appropriately validated for its intended use, balancing the level of validation effort with the criticality and intended application of the assay.
Introduction
B-cell-depleting monoclonal antibodies and mAb-based products are pivotal in treating various autoimmune and oncological diseases. By selectively targeting and eliminating B-cell populations, these therapies help manage conditions associated with abnormal B-cell function or overactivity, including non-Hodgkin lymphoma, chronic lymphocytic leukaemia, rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis. Specific depletion with no significant impact on other immune cells is achieved by binding to the CD20 receptor, an antigen expressed primarily on the surface of B-cells. Five drugs from this group are currently marketed across the EU and the US: rituximab (precursor of anti-CD20 mAbs, approved in 1997), ofatumumab, obinutuzumab, ocrelizumab and ublituximab.
Pharmacodynamics of B-cell-depleting therapeutics is assessed via a combination of direct and indirect methods, including flow cytometry and immunohistochemistry monitoring of B-cells, quantitation of serum biomarkers, PK/PD modelling, and, ultimately, evaluation of clinical endpoints. This multifaceted approach provides a comprehensive understanding of how the tested drug affects target cell populations and overall disease activity, guiding effective treatment strategies and optimizing patient outcomes. Of all listed methods, bioanalytical flow cytometry is considered the gold standard for measuring B-cell depletion and recovery in whole blood after anti-CD20 treatment.
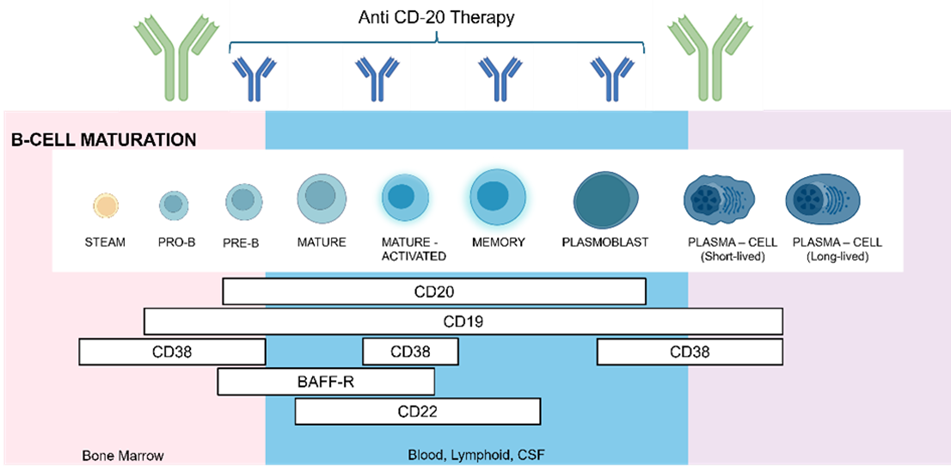
Figure 1 Stages of B cells maturations and expression of cell-surface antigens.
Designing a B-cell depletion bioanalytical flow cytometry assay
One of the most important considerations when designing a B-cell quantification assay is the selection of antigens that will be used to identify the desired population of B lymphocytes. Crucially, the chosen antigen cannot be a therapeutic target, and at the same time, it should be specific to the studied population. Therefore, despite its selective expression, CD20 cannot be used in our assay, as during the anti-CD20 therapy, all receptors are already occupied by administered mAbs, interfering with the binding of fluorescently labelled antibodies.
Like CD20, CD19 receptor is also a B-cell lineage-specific antigen expressed on the cell surface (Figure 1). While CD20 is acquired during the late stages of B-cell lymphogenesis and is then lost upon differentiation into plasma cells, CD19 expression covers the entire spectrum of early B-cell genesis and maturation.1 Because CD19 is restricted to B cells, it serves as a specific marker in bioanalytical flow cytometry, allowing for the accurate identification and enumeration of B cells without cross-reactivity with other immune cells.
The B-cell counting assay developed at Mabion utilizes the IVD-certified TBNK reagent (BD Multitest 6-Color TBNK with BD Trucount™ tubes) to determine the percentages and absolute B cell counts in whole peripheral blood. It can also measure the percentages or absolute counts of T lymphocytes (CD3+), natural killer (NK) lymphocytes (CD3–CD16+ and/or CD56+), helper/Inducer T lymphocytes (CD3+CD4+), and suppressor/cytotoxic T lymphocytes (CD3+CD8+).
However, our laboratory customizes the original method to our intended use by:
- Applying the blood collection tubes that include both anticoagulant (K3EDTA) and cell preservation reagents instead of ‘gold standard’ K3EDTA tubes prolongs the stability of the cell surface antigens and improves the SSC properties of lymphocytes till the maximum time of 14 days from the time of collection.
- Testing whole blood subjects derived from RA (Rheumatoid arthritis) and NHL (non-Hodgkin lymphoma) patients in the presence of anti-CD20 treatment.
Following Clinical and Laboratory Standards Institute guideline H62–Validation of Assays Performed by Flow Cytometry, introducing modifications to IVD-certified bioassays requires a systematic and thorough approach to ensure that the modified assay continues to meet performance standards and regulatory requirements.2 In this context, the use of blood-stabilizing products is a critical change introduced for the assay. First, cell preservative compounds can cross-link, masking the target epitopes. Second, these compounds can interfere with tandem fluorochromes, giving false positive results. By conducting fit-for-purpose validation, our laboratory successfully implemented these modifications, improving assay performance while maintaining results reliability (see section Validation).
TBNK bioanalytical flow cytometry assay performance
The TBNK reagent is composed of seven monoclonal antibodies conjugated to specific fluorochromes (CD45-PrCP-Cy5.5, CD19-APC, CD3-FITC, CD4-PE-Cy7, CD8 APC-Cy7, CD16C-PE D56). The reagent is added to a trucount tube containing a lyophilized pellet of fluorescent beads. Next, the whole blood specimen is directly transferred to the antibodies/beads mix and incubated. During incubation, each monoclonal antibody in the reagent binds to a specific antigen on the surface of blood cells, while beads dissolve, releasing a known number of fluorescent beads. Samples are acquired on a BD bioanalytical flow cytometer (Facs Lyric or FACS Verse) using the predefined protocol on FACS Suite software. The gating strategy of the assay is presented in Figure 2.
After data acquisition, sample gating is reviewed by the analyst and adjusted if required. All predefined protocol changes are automatically recorded in the assay report. Analysts should be guided by Standard Operating Procedure (SOP), which outlines the rules for interpreting and analyzing questionable dot plots. Visual examination of each gate is supported by sample quality questionable error messages, like:
- Not critical, disease-specific parameters that could also be associated with staining error (%T- sum failure – indicates a large number of double-positive or double-negative T cells, % Lymphosum failure – events could be incorrectly classified as T, B, or NK cells).
- Critical, concerning the acquisition stopping criteria parameters (lymph gate does not contain requested 2,500 events, Insufficient beads acquired (<500).
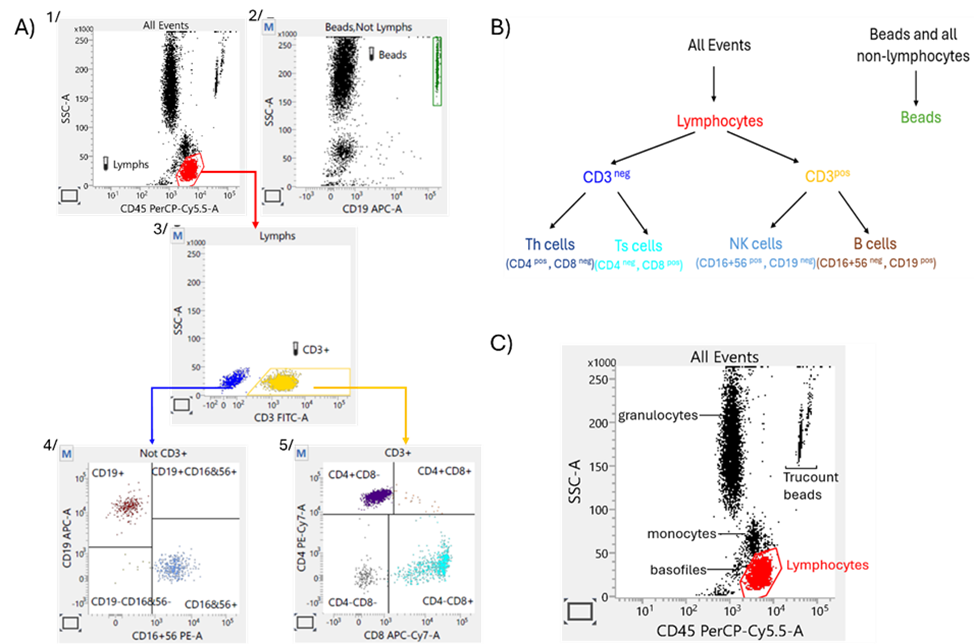
Figure 2 A streamlined methodology for PD assessment of anti-CD20 agents: A) TBNK gating strategy*, B) Population hierarchy, C) Identification of blood cells subpopulation on SSC vs. CD45 plot.
* 1. The software collects 10,000 total events, applies the expert ‘lymph’ gate on the SSC/CD45 plot, and calculates the number of lymphocytes in the gate. The software collects 10,000 total events, applies the expert ‘lymph’ gate on the SSC/CD45 plot, and calculates the number of lymphocytes in the gate. The software continues acquiring until the 2500 number of lymphocytes is reached.
2. The Trucount beads are gating on SSC/CD19 plot (population all events without lymphocytes – Beads, no Lymph).
3. CD3+ lymphocytes are gated on SSC/CD3 plot 5/ CD3+ cells are dividing on CD4+ and CD8+ subpopulation on CD4/CD8 4/ B-cells and NK- defining gates are adjusted on CD19/CD16CD56 plot (presenting non-CD3+lymph event).
Quality control samples
Various biological standards are used for bioanalytical flow cytometry, including preserved whole blood, PBMCs (peripheral blood mononuclear cells), and dried leukocytes. However, only a limited number of standards are provided with certified target values, such as absolute cell counts. If they are certified, the range for target value is extensive, and often, it is not provided for each subset analyzed with a given method. Consequently, they cannot be considered ‘real’ reference standards. The BD™ Multi-Check Control and CD-Chex Plus (Streck) are stabilized human leukocytes and erythrocytes in a preservative medium dedicated to complete process control for TBNK immunophenotyping of whole blood samples. There is a control for antibody staining, red blood cell (RBC) lysis, instrument setup and performance, and data analysis on FACS. The experimental cell number value of the specific lymphocyte subpopulations must be within limits specified by the manufacturer in the certificate of analysis. Following the manufacturer’s recommendations, the biological standard should be analyzed at least once per analytical day. However, some laboratories, including ours, analyze the biological standard parallel to the test samples during each analytical run.
Day-to-day instrument and assay standardization, monitoring and setup
PQC (Performance Quality Control) with CS&T beads (Cytometer Setup and Tracking beads) are used to define the baseline performance of the cytometer. They check laser alignment, measure %rCV, linearity, resolution and laser power, compare PMT voltages to characterization QC (baseline), and update spillover values and PMTVs for default settings. Daily measurements are automatically entered into Levey-Jennings to detect increased random errors and shifts or trends in cytometer performance. This meticulous monitoring of FACS performance measurements over time ensures consistent instrument performance, promptly addresses issues and maintains the quality and reliability of data.
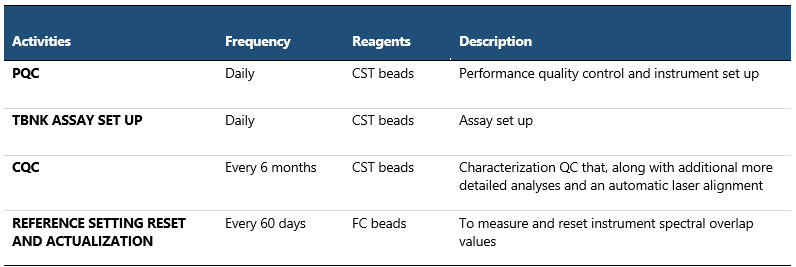
Table 1 Quality Control of Instrument Performance and Instrument Setup
Validation of bioanalytical flow cytometry assay
The CLSI H62 document marks a significant advancement in standardizing flow cytometry assay validation practices, building on the foundational consensus papers published over the last decade7,8. Before its publication, no official guidelines addressed the validation requirements of flow cytometric methods used in drug development or clinical testing.
Due to the complexity of flow cytometry technology, lack of certified reference materials, and different categories of bioanalytical data (qualitative, quantitative, quasi-quantitative), not every validation parameter can be assessed.8 Assay specificity, sensitivity (limits of detection /quantification), precision (repeatability), stability and reference ranges are always validated for the bioanalytical flow cytometry method. Linearity, standard calibration, and interference (matrix, drug) can also be validated in some cases. However, accuracy, selectivity, range of quantification, incurred sample reanalysis, normal signal distribution, and prozone effect usually cannot be evaluated in a direct manner. Furthermore, the specific validation parameters to be assessed depend on the intended data use. If it changes during the assay’s lifetime, additional validation parameters can be addressed at that time. This iterative approach is known as “Fit-for-Purpose” validation.2
The TBNK reagent is used for the measurement of [%] and absolute counts of Ts, Th, B, and NK Cell. However, the CD3–CD19+ B cells are enumerated for pharmacodynamics evaluation of anti-CD20 therapeutics, and consequently, the results obtained for this subpopulation are considered critical for determining assay validity. This assay is classified as quasi-quantitative because the absolute cell count can be determined by comparing cellular events to the certified number of bead events. In Table 2, we present our approach to validating the quasi-quantitative determination of B lymphocytes in human whole blood in the presence of anti-CD20 treatment. For each validation parameter, the acceptance criteria were predefined before the start of validation.
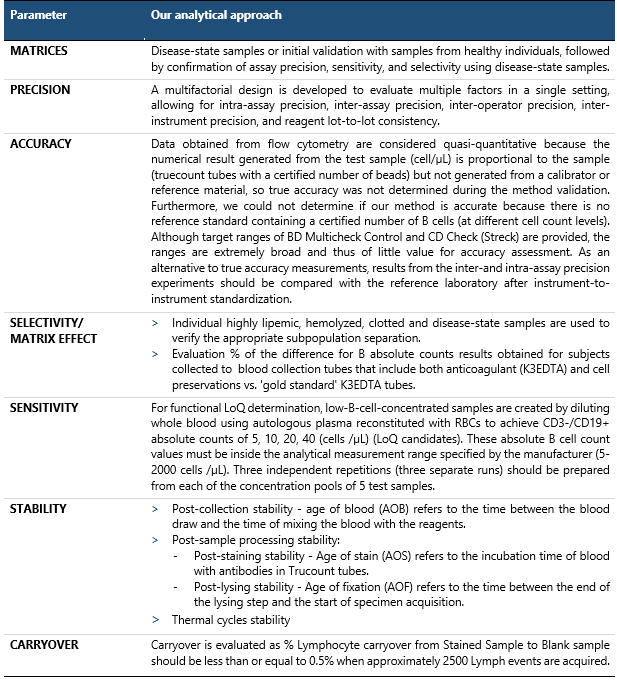
Table 2 Assessment of validation parameters for quasi-quantitative determination of B-lymphocytes in human whole blood in the presence of anti-CD20 treatment
Since we modified the original test protocol using blood-stabilizing products, monitoring the correct separation of the TBNK subpopulation over time was critical during the validation phase. Ageing of test samples can cause both losses of TBNK antigen detection (due to protein degradation) and change in Leucocytes’ morphology (loss of the difference in SSC properties). Both cases could affect the absolute lymphocyte counts’ measurements and inclusion of basophils (less bright CD45, low side scatter) or monocytes (less bright CD45, moderate side scatter) in a lymphocyte cluster.
Our validation data showed that test samples collected in blood collection tubes that include both anticoagulant (K3EDTA) and cell preservation reagents (P2) remain stable at 2-8°C up to 14 days from the day of collection and at RT up to 3 days from the time of collection compared to the baseline sample (collected in gold-standard K3EDTA tubes without preservatives (P1)), as presented in Figure 3. Furthermore, leukocyte subpopulations are adequately separated at any tested point in time.
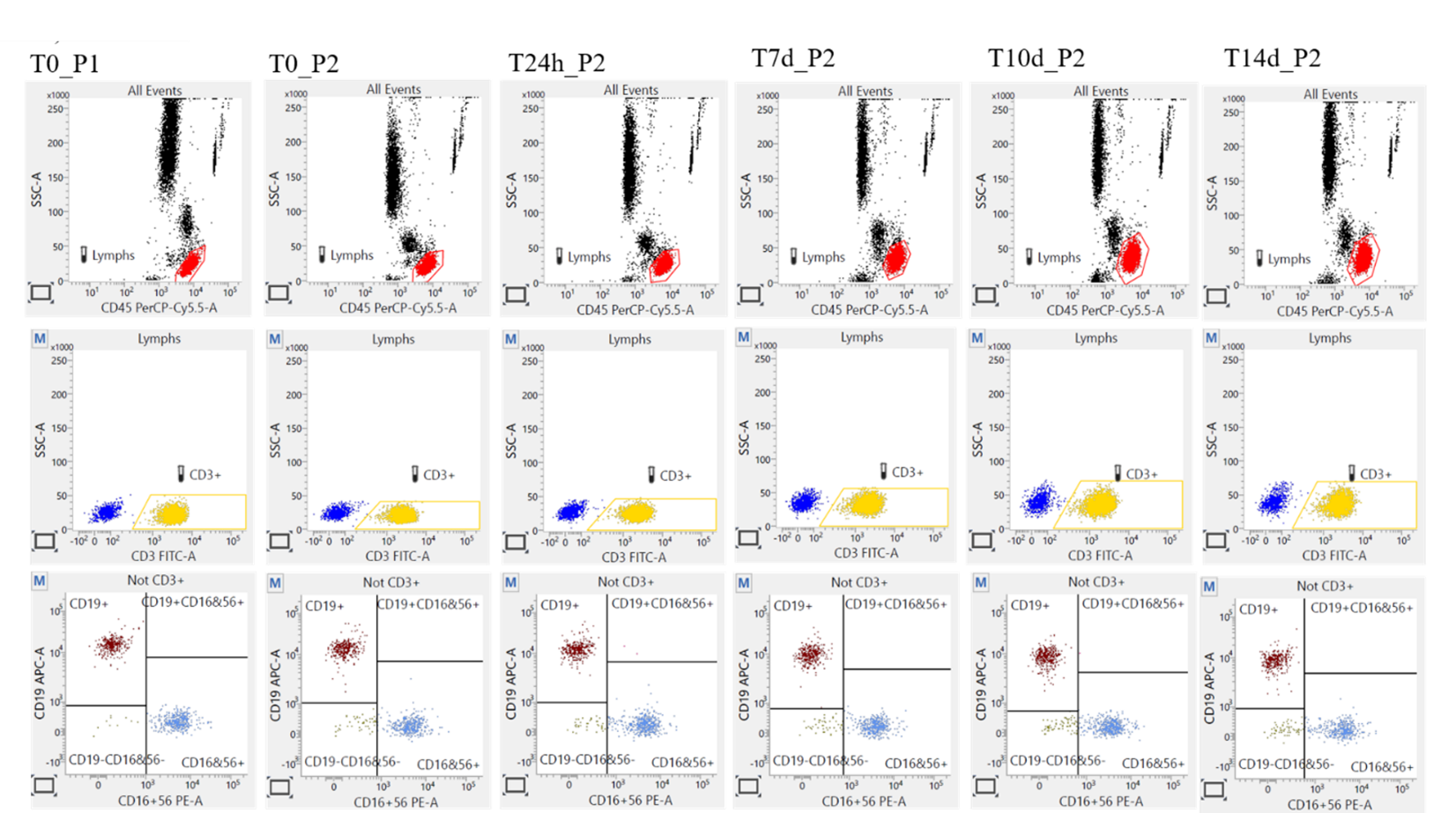
Figure 3 Cell preservatives effect on TBNK subpopulations separation.
The visualization of the lymphocytes tested via cell markers CD45, CD3, CD19, CD16+56 for the control (P1 and P2 <6 hours, Day 0), P2 after 24h, on day 7, and 14, have shown that the Lymphocytes from P1 samples exhibited higher side scatter (SSC) properties than the equivalent cells in the P2 tubes; however, the SSC properties also increase in time (from T0 to 14days) in P2 tubes. On the other hand, the fluorescence characteristics are mostly stable in both time and storage conditions. These results demonstrate that although stabilization affects the cells’ physical properties, the different cell populations can be adequately identified.
Abbreviations: P1 – gold-standard K3EDTA tubes without preservatives; P2 – blood collection tubes that include both anticoagulant (K3EDTA) and cell preservation reagents, Time of testing: T0 (≤6h from the draw), T24 (24h±2h from the draw), T7d (7days±1day from the draw), T10d (10 days±1day from the draw), T14d (14 days±1day from the draw).
Figure 3 shows the lymphocytes’ visualization of DonorX tested via cell markers CD45, CD3, CD19, CD16+56 for the control (P1 and P2 tested <6 hours after drawing, Day 0), P2 after 24h, on day 7, and 14. Lymphocytes from P1 samples exhibited higher side scatter (SSC) properties than the equivalent cells in the P2 tubes, and the SSC properties also increased in time (T0 to 14 days) in P2 tubes. However, the leucocyte subpopulations are adequately separated at any point in time.
After validating an assay with a comprehensive QC process, it’s crucial to continuously monitor and maintain performance to ensure it aligns with the established specifications.
TBNK immunophenotyping assay in the monitoring and management of oncological therapies
TBNK immunophenotyping is an invaluable tool in managing oncological and autoimmune disorders. Developed initially to monitor CD4 T-cell counts in HIV-positive patients9, this assay has evolved significantly, finding critical applications across various medical fields. The ability to quantify and characterize T-cell, B-cell, and NK cell populations provides profound insights into immune system dynamics, aiding in the diagnosis, monitoring, and therapeutic assessment of numerous diseases.
TBNK immunophenotyping can be used for the following purposes:
- Real-time Monitoring: Provides immediate feedback on the immune system’s response to therapy, allowing for rapid adjustments in treatment strategy.
- Mechanistic Insights: Helps in understanding how drugs modulate the immune system, providing insights into their mechanisms of action.
- Predictive Value: Early changes in TBNK profiles can predict long-term outcomes, helping to identify responders and non-responders.
- Safety Management: Identifies potential immune-related adverse events early, allowing for proactive management of toxicities.
Specific examples of TBNK assays’ application include:
- Checkpoint Inhibitors (e.g., Anti-PD-1/PD-L1, Anti-CTLA-4) 10
- Pembrolizumab (Keytruda) and Nivolumab (Opdivo): These anti-PD-1 antibodies are used in the treatment of melanoma, renal cell carcinoma, and non-small cell lung cancer. TBNK immunophenotyping can track the changes in T-cell populations, significantly increasing the number of effector T-cells and decreasing the number of regulatory T-cells, which correlate with therapeutic response.
- Ipilimumab (Yervoy): An anti-CTLA-4 antibody used in the treatment of melanoma and other solid tumors. Monitoring the increase in activated T-cells and reduction in regulatory T-cells helps assess treatment efficacy and manage immune-related adverse events.
- CAR-T Cell Therapy (e.g., Tisagenlecleucel, Axicabtagene Ciloleucel)
- Tisagenlecleucel (Kymriah): Indicated for B-cell acute lymphoblastic leukaemia (ALL) and diffuse large B-cell lymphoma (DLBCL). TBNK immunophenotyping is crucial to track the expansion and persistence of CAR-T cells in the patient’s blood, as well as the depletion of B-cells, which indicates the CAR-T cells’ effectiveness.
- Axicabtagene Ciloleucel (Yescarta): Used for treating DLBCL. Monitoring involves tracking CAR-T cell populations and their impact on normal B-cell counts to manage potential B-cell aplasia.
- Bi-specific T-cell Engagers (BiTEs) (e.g., Blinatumomab).
- Blinatumomab (Blincyto): Used in the treatment of B-cell precursor ALL. TBNK immunophenotyping helps in monitoring the engagement between T-cells and B-cells, indicating the drug’s efficacy in redirecting T-cells to kill B-cell leukaemia cells.
- Monoclonal Antibodies (e.g., Rituximab, Daratumumab)
- Rituximab targets CD20 on B-cells and is used as a treatment for non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukaemia (CLL). TBNK immunophenotyping assesses B-cell depletion and recovery post-therapy.
- Daratumumab (Darzalex): Targets CD38 on plasma cells and is used for treating multiple myeloma. Monitoring involves tracking the depletion of CD38-positive cells and changes in other lymphocyte populations.
- Immune Modulators (e.g., Lenalidomide)
- Lenalidomide (Revlimid): Used as a treatment for multiple myeloma and mantle cell lymphoma. TBNK immunophenotyping helps evaluate the drug’s effect on enhancing T-cell and NK cell activity while reducing regulatory T-cells, contributing to its anti-tumour effects.
Summary
Flow cytometry assays have a variety of intended purposes that include basic research, drug discovery, and the exploratory, secondary, and primary endpoint of clinical trials. In designing a fit-for-purpose validation for a B cell-depleting assay, it is crucial to tailor the validation experiments to meet the specific requirements of the assay’s intended use. This approach ensures that the assay delivers reliable, “accurate” results and is efficient and practical. Several parameters, such as accuracy, sensitivity, and selectivity, pose significant challenges in validating a TBNK assay, especially when dealing with quasi-quantitative data. This paper describes the solutions employed in our laboratory to address these challenges effectively. Remember:
- Any modifications made by the laboratory to the assay manufactured as IVD’s instructions will render the test no longer FDA-cleared/approved. The laboratory must verify/validate these modifications in terms of the intended use.
- In clinical trials, the use of solutions that effectively extend the ‘lifetime’ of the test sample is not just a necessity, but a critical need, given the time-sensitive nature of the trials and the need for reliable data.
- Validation in bioanalytical flow cytometry is not straightforward; each validation parameter’s experiment is designed based on the purpose of the test. There is no universal design for all methods, but we have developed a specific approach that we have found to be effective. You can refer to Table 3 for a detailed description of our specific approach.
If you are interested in this publication or would like to learn more about bioanalytical flow cytometry methods, please get in touch with us to collaborate. We also invite you to visit our dedicated analytics section to learn about our other analytics methods.
Prepared by:
Beata Talar
References
- Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006 May;6(5):394-403. doi: 10.1038/nri1838.
- CLSI, Clinical and Laboratory Standards Institute. (2021). H62 Validation of Assays Performed by Flow Cytometry. Available at: https://clsi.org/standards/products/hematology/documents/h62/ (Accessed September 1, 2024).
- der Strate BV, Longdin R, Geerlings M, Bachmayer N, Cavallin M, Litwin V, Patel M, Passe-Coutrin W, Schoelch C, Companjen A, Fjording MS. Best practices in performing flow cytometry in a regulated environment: feedback from experience within the European Bioanalysis Forum. Bioanalysis. 2017 Aug;9(16):1253-1264. doi: 10.4155/bio-2017-0093.
- Green CL, Brown L, Stewart JJ, Xu Y, Litwin V, Mc Closkey TW. Recommendations for the validation of flow cytometric testing during drug development: I instrumentation. J Immunol Methods. 2011 Jan 5;363(2):104-19. doi: 10.1016/j.jim.2010.07.004.
- O’Hara DM, Xu Y, Liang Z, Reddy MP, Wu DY, Litwin V. Recommendations for the validation of flow cytometric testing during drug development: II assays. J Immunol Methods. 2011 Jan 5;363(2):120-34. doi: 10.1016/j.jim.2010.09.036.
- Johansson U, Bloxham D, Couzens S, Jesson J, Morilla R, Erber W, Macey M; British Committee for Standards in Haematology. Guidelines on the use of multicolour flow cytometry in the diagnosis of haematological neoplasms. British Committee for Standards in Haematology. Br J Haematol. 2014 May;165(4):455-88. doi: 10.1111/bjh.12789.
- Stevenson L, et al., 2018 White Paper on Recent Issues in Bioanalysis: focus on flow cytometry, gene therapy, cut points and key clarifications on BAV (Part 3 – LBA/cell-based assays: immunogenicity, biomarkers and PK assays). Bioanalysis. 2018 Dec;10(24):1973-2001. doi: 10.4155/bio-2018-0287.
- Selliah N, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, Litwin V. Flow Cytometry Method Validation Protocols. Curr Protoc Cytom. 2019 Jan;87(1):e53. doi: 10.1002/cpcy.53.
- Omana-Zapata I, et. al., Accurate and reproducible enumeration of T-, B-, and NK lymphocytes using the BD FACSLyric 10-color system: A multisite clinical evaluation. PLoS One. 2019 Jan 28;14(1):e0211207. doi: 10.1371/journal.pone.0211207.
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021 Sep 11;398(10304):1002-1014. doi: 10.1016/S0140-6736(21)01206-X.
Related resources
Time to Market Strategy – 5 CDMO Tactics to Speed Up Biologic Launches
Biologics, Drug development, Manufacturing

Navigating OOX Results: Effective Analysis and Management in CDMO Laboratories
Analytics, Manufacturing, Regulatory

Mammalian cell lines as a platform for protein vaccine production
Cell culture, Drug substance, Manufacturing, Proteins, Vaccines