Innovative biologics – expected drug approvals in 2025
Antibody-drug conjugates, Biologics, Bispecific antibody, Clinical trials, EMA, FDA, Monoclonal antibody, Vaccines
- A huge wave of innovative biologics is anticipated to gain approval in 2025, continuing the upward trend observed in recent years. Among the potential blockbusters are new gene therapies, interfering RNAs and antibody-drug conjugates (ADCs), some of which are first-in-class molecules with entirely novel mechanisms of action.
- In this article we delve into 14 new biologic drugs and vaccines, highlighting the most promising candidates that are poised to shape the medical landscape in the coming years. These include among others: fitusiran (an siRNA therapy for hemophilia), clesrovimab (an anti-RSV antibody) and nipocalimab (FcRN-targeting mAb for myasthenia gravis).
- In 2025 we are also likely to witness the approval of two new vaccines, including mRNA-1010 (an mRNA-based influenza vaccine) and MenABCWY (a pentavalent meningococcal vaccine).
Last year, the FDA granted approval to 50 new medicines, of which 16 (32%) were biologics.1 These numbers are only slightly lower than in two records years of 2018 and 2023, when 17 biologic drugs were approved. The bar is set high, but all the signs indicate that the current year will be not worse than the previous ones. Tens of innovative biologics are already in regulatory review or in the queue for filing a marketing application. Here, we present a list of the most exciting biologic drugs and vaccines anticipated to receive approval in 2025:

Brand new antibodies and fusion proteins
Lerodalcibep
For many years, pharmaceutical companies have been looking for new cholesterol-lowering drugs that would complement or even supersede the commonly used statins, allowing to further reduce the level of this important risk factor. After several spectacular and costly failures e.g., CETP inhibitors, which showed no additional decrease in cardiovascular events, a new family of medicines capable of lowering both cholesterol and heart attack incidence, has been finally discovered. This family, called PCSK9 (proprotein convertase subtilisin/nexin type 9) inhibitors, uses a different mechanism of action to achieve the same goal as statins: get LDL-C (so called “bad” cholesterol) out of the bloodstream to halt the development of atherosclerotic plaques. Whereas the older drugs increase the expression of LDL-C receptors at hepatocytes surface, PCSK9 inhibitors act from the opposite side, preventing their degradation and thereby accelerating the process of cholesterol removal. This new pharmacologic class, consisting of several approved monoclonal antibodies such as alirocumab (Praulent) and evolocumab (Repatha), can lower blood LDL-C levels by as much as 62%, a result that can hardly be achieved even with the highest statin dose. The addition of PCSK9 inhibitor to usual lipid lowering management has been shown to reduce the risk of repeat heart attacks and strokes in patients with established cardiovascular disease.2
The success of alirocumab and evolocumab, and high prevalence of the conditions they treat, prompted other drug-makers to develop their own PCSK9-targeting molecules.3 One of such molecules is lerodalcibep developed by LIB Therapeutics, which has recently completed the pivotal Phase III trial LIBerate-HR and submitted a BLA.4 Unlike all other PCSK9 inhibitors, which are monoclonal antibodies, lerodalcibep is a recombinant fusion protein combining an anti-PCSK9 protein called adnectin with human serum albumin.5 Although the mechanism of removing LDL-C is similar to other members of this class i.e., preventing break down of LDL receptors by PCSK9, the fusion with albumin increases the protein stability at ambient temperatures, allowing for easier storage and convenient once-monthly administration.
In the LIBerate-HR trial, enrolling 922 patients with or at high risk of cardiovascular disease, lerodalcibep demonstrated a 56% reduction in LDL-C levels compared to placebo over 52 weeks.6 Additionally, 90% of participants achieved both a ≥50% reduction in LDL-C and met guideline-recommended targets. The safety profile was comparable to placebo, with mild injection site reactions being the most common adverse events. There was also a trend towards lower incidence of cardiovascular events in the lerodalcibep group (5.7% vs. 7.8%).
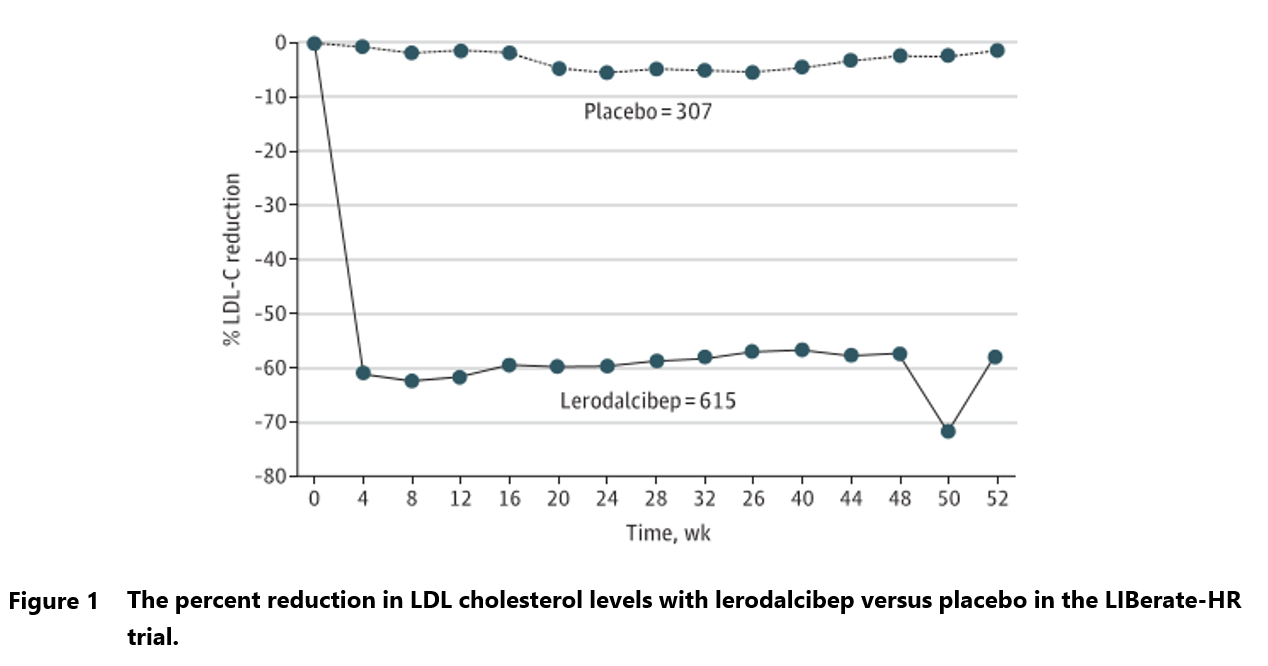
Lerodalcibep approval would offer yet another therapeutic option for patients with hypercholesterolemia and those at high risk for cardiovascular events who require additional LDL-C lowering despite maximally tolerated statin therapy.7 The price of the new drug will likely be comparable with Praulent and Repatha, which offer similar reduction in cholesterol levels. Therefore, that innovative biologics’ commercial success will be hinged on physicians’ and patients’ consideration of benefits from once monthly administration and lack of requirement for refrigeration.
Apitegromab (SRK-015)
Another interesting candidate for innovative biologics of 2025 is apitegromab, a fully human monoclonal antibody designed to inhibit the activation of myostatin and improve the symptoms of spinal muscular atrophy (SMA).8 SMA is a rare genetic neuromuscular disorder characterized by the degeneration of motor neurons, leading to muscle weakness and atrophy. Myostatin plays a critical role as a negative regulator of muscle growth.9 By inhibiting the activity of this protein, apitegromab aims to promote muscle growth, increase motor function and improve the quality of life of patients with SMA.
Apitegromab was recently reported to have met primary endpoint in a double-blind, placebo-controlled phase 3 trial in patients concurrently treated with approved SMA therapies.10 Nearly a third (30.4%) of participants receiving apitegromab had a >3-point improvement on HFMSE, a gold standard scale for assessing motor function. The proportion of responders in placebo group was much lower (12.5%), the difference being statistically and clinically significant. Innovative biologics benefit could be observed already at 8 weeks, meaning that patients would not have to wait long before noticing improvement in their daily activities and mobility.
The new drug fills an important gap in SMA treatment which persist despite the availability of effective therapies such as nusinersen (Spinraza), risdiplam (Evrysdi) or onasemnogene abeparvovec (Zolgensma). All these innovative biologics, while preventing the degenerative course, have limited efficacy when it comes to treating muscle impairment arising from the pre-existing neurodegeneration. This creates an area where myostatin inhibitor could present much added value, allowing SMA patients to regain some of the lost capacities.11 As apitegromab is intended to be used on the top of existing therapies, offering patients some unique advantages, it should not face a significant competition after approval. The developer of apitegromab, Scholar Rock has already applied for marketing authorization in the US and the EU application will soon follow.12
Depemokimab
Depemokimab, a long-acting IL-5-targeting mAb developed by GSK, is likely to become a new blockbuster.13 The innovative biologics antibody was designed to provide a novel option for patients suffering from uncontrolled asthma and other inflammatory diseases such as rhinosinusitis with nasal polyps.14 As millions of people around the world are struggling with these conditions and depemokimab has shown truly impressive results in clinical trials, it is well positioned for a commercial success.
The mechanism of action of depemokimab is simple. By blocking IL-5 interaction with its receptor, it inhibits the activity of this cytokine that promotes eosinophil activation and survival. By reducing eosinophil levels, the novel mAb aims to alleviate inflammation and associated symptoms in patients with severe asthma and other related conditions.14 The benefits of depemokimab were amply shown in SWIFT-1 and SWIFT-2 trials.15 Large reductions (48%-54%) in the rate of clinically significant asthma exacerbations were demonstrated during 52 weeks of treatment (Table 1). However, the innovative biologics had little effect on everyday asthma symptoms, which can be partially attributed to intense background therapy with corticosteroids. Number of patients with adverse events on depemokimab was similar to placebo. Similarly positive results were also seen in rhinosinusitis trials, where depemokimab significantly reduced nasal polyp size and alleviated nasal obstruction.16
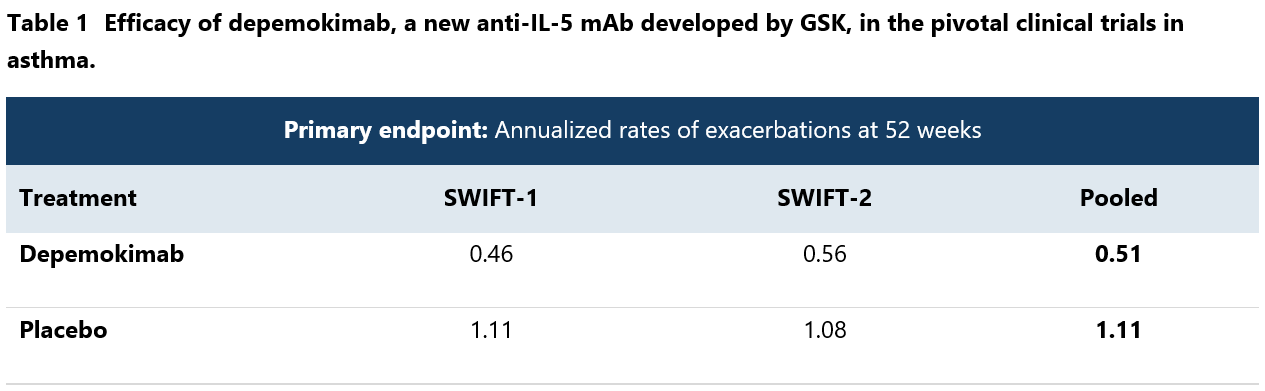
In addition to efficacy, the new IL-5 inhibitor has an advantage of extended half-life, allowing for administration once every 6 months. Along with subcutaneous route of administration, it provides patients with a convenient option to halve their risk of severe asthma attacks.
The GSK plan is to have a dual indication launch this year, in severe eosinophilic asthma and chronic rhinosinusitis.13 Applications will be first submitted to FDA, followed by authorities in the EU, China and Japan. Trials in other inflammatory indications are ongoing and the list of depemokimab’s indications will likely grow with time
Clesrovimab
Nirsevimab (Beyfortus), AstraZeneca’s highly successful anti-RSV antibody, will soon have to face competition from its first rival, clesrovimab, developed by Merck. The clinical benefits of the novel drug are as clear-cut and similar to its predecessor. Clesrovimab was found to decrease the risk of severe RSV-associated lower respiratory infection by a whooping 91.7%, with only 2 cases among 2,398 treated children occurring during the 6-month follow-up period. Even when counting all-cause hospitalizations for lower respiratory infection, the risk reduction was still impressive (49.0%).17
Clesrovimab and nirsevimab work in essentially the same way. The innovative biologics both bind to RSV F protein, which is used by the virus for cell entry. Introduction of several mutations afforded clesrovimab with a long half-life, that allows to obtain a full protection during the whole RSV season with only a single administration.18
Passive immunization with anti-RSV antibodies has been widely implemented across the European Union and US. Countries such as Spain or France are now routinely administering nirsevimab prior to the RSV season to nearly all newborn children as part of the state-sponsored prophylaxis program. The result was a drastic fall in hospitalizations for bronchiolitis, a dreadful complication of infection with this virus, in recent years.19 Unfortunately, the huge demand for nirsevimab shortly after its approval resulted in widespread shortages. This is where clesrovimab could step in. By quickly launching the commercial production and offering the RSV prophylaxis to other EU countries, Merck would have a chance to gain a significant portion of the market despite the presence of a strong competitor.
Clesrovimab MAA is currently evaluated by the EMA and FDA, with drug launch anticipated in the 2nd half of 2025.20
Nipocalimab
Another innovative biologics candidate for approval in 2025 is nipocalimab, a monospecific mAb designed to treat a variety of IgG antibody-mediated autoimmune diseases.13 Originally developed by Momenta Pharmaceuticals, nipocalimab is now under development by Janssen Research & Development and Johnson & Johnson.
Nipocalimab binds with high affinity to block the neonatal Fc receptor (FcRn). By blocking FcRn, nipocalimab reduces levels of circulating immunoglobulin G (IgG) antibodies, including autoantibodies and alloantibodies, with little impact on other immune functions.13 This mechanism of innovative biologics is obviously relevant for the treatment of various autoimmune diseases. And indeed, the approval for nipocalimab is pursued in several such disorders, including autoimmune hemolytic anemia (AIHA), hemolytic disease of the newborn, neonatal autoimmune thrombocytopenia, chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and, most importantly, myasthenia gravis, which is already under priority review at the FDA (BLA submitted in August 2024).21 Phase III trials are in progress for the other listed conditions.9 Additionally, the drug is being studied for Sjogren’s syndrome, myositis, rheumatoid arthritis, and systemic lupus erythematosus.
A phase 3 Vivacity-MG3 trial in generalized myasthenia gravis showed sustained disease control over 24 weeks in antibody-positive participants.22 Outcomes were superior in participants who received nipocalimab plus standard of care compared to those who received placebo plus standard of care. In Sjögren’s Disease, DAHLIAS study demonstrated a greater than 70% relative improvement in systemic disease activity at Week 24 in participants treated with nipocalimab 15 mg/kg compared to placebo.23 When it comes to hemolytic disease of the fetus and newborn (HDFN), Phase 2 UNITY study showed that nipocalimab delayed or prevented severe fetal anemia, and 54% of study participants achieved a live birth.24 Interestingly, despite the potent lowering of antibodies’ concentrations, the investigational FcRN blocker was shown to be remarkably safe with no perceivable increase in the number or severity of adverse events, including serious infections. All data indicate that nipocalimab should receive green light from the FDA this year.
Next wave of antibody-drug conjugates (ADCs)
Telisotuzumab vedotin (ABBV-399)
Telisotuzumab vedotin is one of the three antibody-drug conjugates set for approval in 2025. It is designed to target tumor cells expressing c-Met receptor, a validated therapeutic target already employed by two FDA-approved small-molecule drugs (crizotinib and cabozantinib).13, 25 This innovative biologics consists of c-Met-binding monoclonal antibody linked via a protease-cleavable linker to MMAE, a potent cytotoxic agent. Upon binding to c-Met receptor, the ADC-receptor complex is internalized into the tumor cell, where cellular proteases cleave the linker and release MMAE into the cytoplasm. Free MMAE disrupts the microtubule assembly, leading to cell death. As with other ADCs, targeted delivery of cytotoxin allows to partially spare healthy tissues, minimizing the systemic toxicity and increasing the maximum tolerated dose.
Telisotuzumab vedotin is investigated as a treatment for advanced non-squamous lung cancer (NSCLC) with c-Met overexpression. Abbvie Inc., developer of telisotuzumab vedotin, submitted BLA to the FDA in September 2024. The BLA submission was based on the data from Phase 2 trial (LUMINOSITY), which revealed a significant response rate of 35% and 23% in patients with high and intermediate c-Met levels, respectively.26
Unfortunately, despite the targeted delivery mode and limited systemic exposure to MMAE, this extremely toxic compound is still capable of causing severe adverse reactions from multiple body systems.27 The most concerning is peripheral neuropathy, which is an expected consequence of microtubule assembly inhibition. However, this and other side effects of telisotuzumab vedotin are considered manageable, with temporary discontinuation and dose adjustment implemented as necessary based on patient’s tolerance. In light of the limited treatment options available for patients with c-Met expressing lung cancer, safety profile will certainly not interfere with the approval of this new ADC.
Telisotuzumab vedotin once again underscores the importance of precision medicine, where therapies are decided based on individual’s biomarkers. As c-Met expression is crucial for its effectiveness, a companion diagnostic will be required in each case prior to starting the treatment.
Datopotamab deruxtecan (Dato-DXd)
Datopotamab deruxtecan (Dato-DXd) is an antibody-drug conjugate (ADC) designed to target and treat various epithelial tumors that express Trophoblast cell surface antigen 2 (TROP2).28 This antigen is often highly expressed in tumors and is associated with a poor prognosis, making it a valuable target for cancer innovative biologics therapy, as evidenced by the success of already approved anti-TROP2 antibody, sacituzumab govitecan.29
Datopotamab deruxtecan is composed of a humanized anti-TROP2 monoclonal antibody linked to a topoisomerase I inhibitor payload, which causes DNA damage and apoptosis in TROP2-expressing tumor cells.13, 30 Interestingly, its design incorporates a cleavable linker to reduce systemic exposure and off-target adverse effects. The drug was discovered by Daiichi Sankyo and developed in collaboration with AstraZeneca.
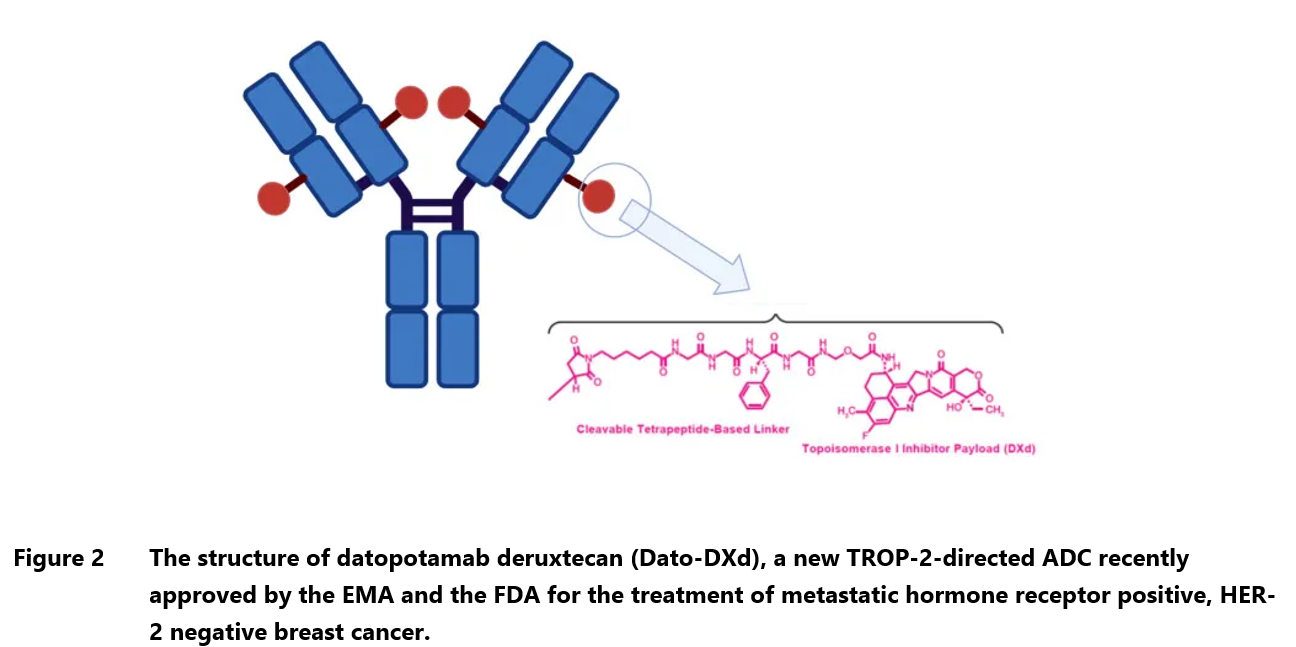
The new ADC has already been approved by the EMA and FDA for the treatment of adult patients with unresectable or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer who have previously undergone endocrine-based therapy and chemotherapy.31 It is also under investigation for non-small cell lung cancer (NSCLC), however, in December 2024 AstraZeneca decided to withdraw lung cancer application in the EU following the receipt of the final list of questions, which questioned the drug’s efficacy. The EMA reviewed the data from TROPION-Lung01 trial, which showed increase in progression-free survival but not overall survival, and considered the evidence insufficient for approval. In the US, datopotamab deruxtecan was granted Breakthrough Therapy Designation in NSCLC and the likelihood of approval is considered higher than in the EU.
In a clinical trial setting, datopotamab deruxtecan demonstrated promising clinical activity and a manageable safety profile in patients with heavily pretreated advanced HR+/HER2- breast cancer and triple-negative breast cancer (TNBC). The objective response rate was 26.8% for HR+/HER2- BC and 31.8% for TNBC.32 As mentioned above, in NSCLC patients datopotamab deruxtecan showed a statistically significant improvement in progression-free survival with fewer discontinuations than with standard chemotherapy.
Patritumab deruxtecan (HER3-DXd)
Another ADC innovative biologics close to obtaining marketing authorization is patritumab deruxtecan (HER3-DXd), which aims at a different molecular target than Dato-DXd but contains identical payload – topoisomerase I inhibitor.13 Patritumab deruxtecan is a fully human ADC designed to combat tumors that express human epidermal growth factor receptor 3 (HER3), being first medication in this class. It contains the same tetrapeptide-based cleavable linkers as Dato-DXd, which limits its toxicity. The drug was discovered by Daiichi Sankyo and is being developed jointly with Merck.
HER3 is highly expressed in breast cancer and other solid tumors and associated with poor prognoses, which makes it a promising therapeutic target.33 It is hoped that patritumab deruxtecan will help treat highly resistant cancers, including heavily pre-treated non-small cell lung cancer (NSCLC), breast cancer, colorectal cancer, biliary cancer, and gastrointestinal cancer. So far, the drug has demonstrated significant activity in NSCLC and breast cancer. In the HERTHENA-Lung01 trial, which was performed in patients with EGFR-mutated advanced NSCLC with progression after two lines of therapy, the objective response rate to HER3-DXd was 29.8% with median duration of response of 6.4 months.34 The drug’s superiority to chemotherapy has been recently confirmed in a larger Phase 3 study, HERTHENA-Lung02, where HER3-DXd was shown to increase the time to disease progression.35 Positive results were also announced in advanced breast cancer across a wide range of HER3 expression levels. In patients with HR-positive/HER2-negative breast cancer, the objective response rate was 30.1% and median progression-free survival (mPFS) was 7.4 months.36 In patients with triple-negative breast cancer (TNBC), a highly resistant tumor with a limited number of treatment options, the ORR was 22.6% and mPFS was 5.5 months.37
Approval of patritumab deruxtecan would mark it as the first HER3-targeted therapy. BLAs have been already submitted to the EMA and FDA.
Game-changing gene therapy for hither to untreatable genetic disorder
Mozafancogene autotemcel (RP-L102)
Now let’s explore the latest developments in the field of gene therapies. As of December 2024, there are 41 cellular and gene therapy products licensed in the US, and this number continues to grow each year.38 There is also a notable increase in the number of newly initiated trials, with 2023 setting an all-time record of 117 clinical trial approvals.39 Certainly, many new gene therapy products will be launched on the market over the next few years.
One of these products will probably be mozafancogene autotemcel (another beautifully complicated name, so in the following paragraphs we will use one of its abbreviations: RP-L102 or fanca-cel). It was developed by Rocket Pharmaceuticals for the treatment of Fanconi anemia complementation group A (FA-A), a rare inherited disorder characterized by early appearing defects of bone narrow and increased risk of hematologic cancer.40 FA-A is caused by multiple mutations in FANCA gene, involved in DNA cross-link repair mechanism and maintenance of normal chromosome stability.41 Fanca-cel delivers a functional copy of FANCA gene into the hematopoietic stem and progenitor cells (HSPCs) collected from a patient by transducing them with a lentiviral vector containing the correct gene sequence.42 Currently, first-line therapy consists or hematopoietic growth factors and androgens, although a more permanent disease remission can be achieved by conventional transplantation of hematopoietic stem cells. However, this option requires a genetically compatible donor. Fanca-cel overcomes this limitation as the cure consists of patient’s own cells.
Fanca-cel has been already evaluated in global clinical studies enrolling 12 pediatric patients with FA-A.42 Transduced stem cells were successfully engrafted in the majority of subjects resulting in functional correction of hematopoietic cells, as judged by their increased resistance to a DNA-damaging agent called mitomycin-c. Hemoglobin, leukocytes and platelet levels were stabilized, showing no progressive fall characteristic for FA-A. No safety issues other than one serious infusion-related reaction were encountered. This event has completely resolved and did not cause any sequalae.
In April 2024, Rocket Pharmaceuticals announced submission of innovative biologics MAA to the European Medicines Agency and in November the company started a rolling application at FDA.43, 44 Approval in both jurisdictions is anticipated in 2025.13 Rocket Pharmaceuticals also develops gene therapies for other incapacitating genetic disorders, including leukocyte adhesion deficiency-I, Danon disease and pyruvate kinase deficiency.43, 45
Another bispecific to watch this year
Denecimig (Mim8)
Mim8, also known as denecimig, is an investigational bispecific antibody designed to mimic activated factor VIII (FVIIIa), whose inborn deficiency causes hemophilia A – a life-threatening blood clotting disorder.46 Administration of Mim8 would correct for this deficiency and protect affected patients from serious bleeding episodes., irrespective of inhibitor status. The innovative biologics developed by Novo Nordisk aims to offer flexible dosing options, including once-weekly and once-monthly subcutaneous administrations.
Mim8 is a fully human antibody which works by bridging the two essential components of blood clotting cascade factor IXa and factor X, emulating the function of the missing FVIII.47, 48 Restored interaction between factor IXa and X leads to activation of the latter, which in turn increases the generation of thrombin and improves blood clotting.
In clinical studies, Mim8 exhibited a favorable PK profile, supporting flexible dosing regimens, including once-weekly and once-monthly subcutaneous administrations.46 The PD assessments of innovative biologics indicated that the drug effectively restored thrombin generation capacity in patients with hemophilia A.49
The pivotal Phase 3 trial FRONTIER2, which compared once-weekly and once-monthly Mim8 with no prophylaxis demonstrated almost complete elimination of treated bleeding episodes in the experimental group (97%-99%).50 The 86% of subjects treated weekly and 95% of those treated monthly experienced no significant bleeds, in contrast to 0% of those without prophylaxis. Mim8 was generally well-tolerated across clinical trials, with no significant safety concerns reported. The incidence of injection site reactions was low, and no thromboembolic events or related serious adverse events were observed.
The unquestionable advantages of denecimig as innovative biologics are its outstanding effectiveness, favorable safety profile and flexible administration (once-weekly, once every two weeks and once-monthly), which allows to provide tailored treatment to meet diverse needs of hemophilia A patients. Its developer, Novo Nordisk, plans to soon apply innovative biologics for regulatory approval in both EU and US.
RNA medicines on the rise
Fitusiran
Many breakthrough therapies for hemophilia emerged in the last decade, including bispecific antibodies (emicizumab and denecimig, see above), gene therapies (etranacogene dezaparvovec, valoctocogene roxaparvovec) and long-acting recombinant coagulation factors (efanesoctocog alfa).51 The most recently approved drug was marstacimab (Hympavzi), a monoclonal antibody directed against tissue factor pathway inhibitor (TFPI).52 All these innovative biologics treatments eliminate the need for frequent clotting factor infusions and reduce the risk of serious bleeding episodes to almost null.
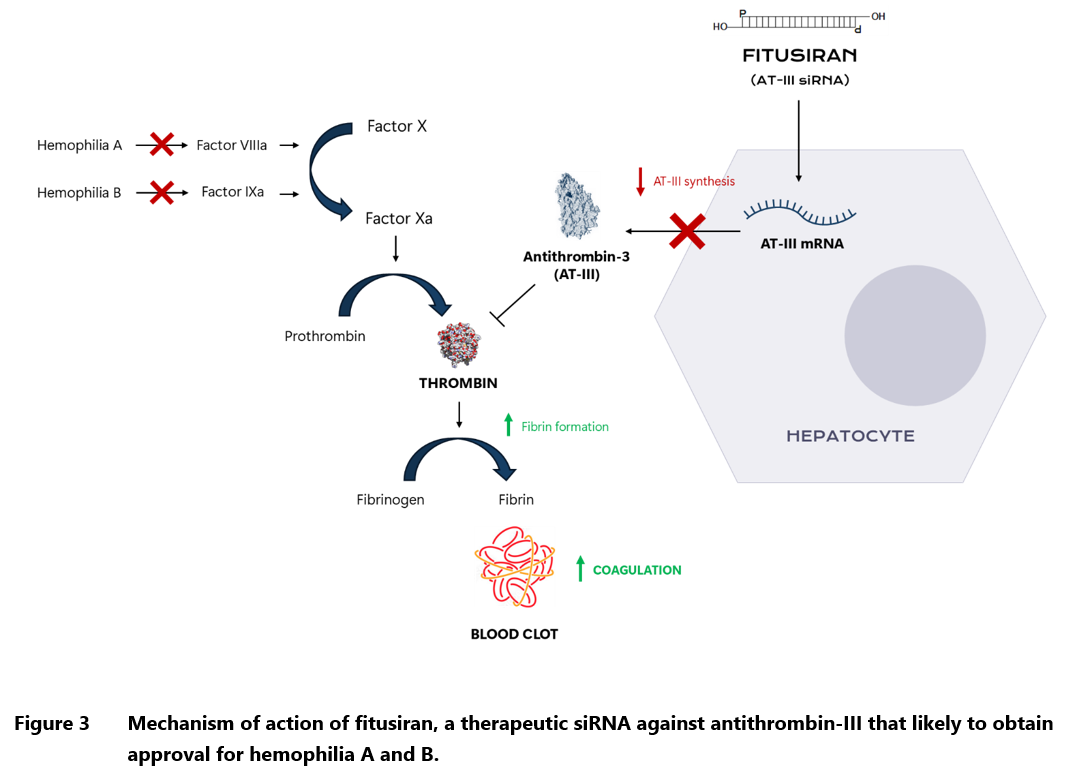
In 2025, another innovative biologics is likely to win the FDA approval. The new drug, fitusiran, is the first ever therapy for hemophilia based on siRNA technology, which works by digesting the complementary mRNA and preventing expression of the specific gene.53 In this case, the target sequence codes for antithrombin, a natural anticoagulant produced in the liver. By lowering antithrombin levels, fitusiran enhances thrombin generation, thereby rebalancing hemostasis and reducing bleeding tendencies in hemophiliacs. If approved, fitusiran would become the second agent that can be used both in hemophilia A and B, regardless of the presence of inhibitors (antibodies against the clotting factors). Patients treated with fitusiran in the pivotal Phase III trial, had a median annualized bleeding rate of 0.0 (0.0–3.4), with 51% having no medically significant bleeding episodes throughout the 8-month observation period. The corresponding results in the control group receiving on-demand clotting factor concentrates were 21.8 (8.4-41.0) and 5%.54 There were no cases of treatment-related thrombosis or any other dangerous side effects, with most adverse reactions being confined to injection site.
The arsenal of hemophilia innovative biologics has been greatly expanded in the recent years, but there is still much room for the new treatment options. Fitusiran is likely to be commercially successful as it can be used in a broad population of patients suffering from both types of hemophilia, regardless of the inhibitor status. The FDA is currently reviewing the drug application and fitusiran approval is expected in March 2025.55
Plozasiran
Next drug on the innovative biologics list is plozasiran, previously known as ARO-APOC3, which is an investigational RNA interference therapeutic developed by Arrowhead Pharmaceuticals.56 Plozasiran development is a culmination of efforts to create targeted treatments for lipid disorders, focusing on those that involve elevated triglyceride levels. These include a common metabolic disorder known as mixed hyperlipidemia as well as an ultrarare genetic disease called familial chylomicronemia syndrome (FCS), which is characterized by excessive accumulation of triglycerides due to mutations in lipoprotein lipase. Regardless of the cause, prolonged hypertriglyceridemia can lead to devastating complications such as severe pancreatitis, steatohepatitis and ischemic cardiovascular disease. The primary goal of innovative biologics therapy is to correct the lipid profile and reduce the incidence of these complications.
Plozasiran achieves that by silencing the production of apolipoprotein C-III (APOC3), which is a key regulator of triglyceride metabolism that inhibits the breakdown of triglyceride-rich lipoproteins (VLDL and chylomicrons) by lipoprotein lipase and decreases their uptake by the liver.56-58 The end result of increased APOC3 activity is thus a significant rise in triglyceride levels. Conversely, decreased synthesis of APOC3 achieved by innovative biologics such as plozasiran leads to normalization of the lipid profiles.
Clinical trial results have shown impressive efficacy for plozasiran. In the Phase 2 SHASTA-2 study enrolling adults with severe hypertriglyceridemia, it demonstrated significant, dose-dependent reductions in atherogenic triglycerides, non-high-density lipoprotein cholesterol (non-HDL-C) and remnant cholesterol. A total of 90.6% of treated patients achieved a triglyceride level of less than 500 mg/dL at the study conclusion.57 Similarly spectacular results of innovative biologics were achieved in mixed hyperlipidemia trial.58 Beneficial impact of plozasiran on both biochemical and clinical outcomes was confirmed in Phase 3 PALISADE study, which evaluated the drug in FCS patients. An 80% decrease in triglycerides led to significant 83% reduction in the risk of acute pancreatitis.55 Plozasiran was well tolerated in all trials with low rates of discontinuation due to adverse events.57-59 Overall, these results overwhelmingly support the drug’s potential effectiveness in managing severe lipid disorders.
Market potential for plozasiran is significant due to the limited treatment options currently available for FCS and other severe hyperlipidemic conditions. The global market for lipid-lowering therapies continues to grow, driven by increasing awareness of cardiovascular diseases and metabolic disorders. High effectiveness of plozasiran positions it uniquely within this market, potentially allowing it to capture a substantial share. Commercial success of this drug depends largely on the results of Phase III cardiovascular outcomes study, CAPITAN, which was recently announced by Arrowhead Pharmaceuticals.60 If plozasiran is indeed shown to reduce the risk of heart disease and stroke in patients with mixed hyperlipidemia, its sales would likely skyrocket.
But first, the innovative biologics must be cleared by regulatory bodies. The company has already submitted FDA application for the treatment of FCS, but as of writing this article, applications for other indications are still pending.61
Boosting immunity with next-generation vaccines
MenABCWY (GSK3536819A)
Hidden under the code GSK3536819A is MenABCWY vaccine, a 5-in-1 meningococcal vaccine candidate developed by GlaxoSmithKline (GSK) to provide a broad protection against the most common serogroups of Neisseria meningitidis: A, B, C, W, Y.62 Together, these serogroups constitute up to 90% of all meningococcal disease cases.63 Until recently, we only had vaccines covering separately MenB (Bexsero, Trumenba) and MenACWY (Menveo, MenQuadfi and Nimenrix).60 This changed in October 2023, when the FDA licensed Pfizer’s meningococcal vaccine, Penbraya, opening up an era of 5-in-1 combination vaccines against meningococcal disease. As Sanofi is also pushing the development of its own MenABCWY candidate (currently in Phase 2 trials), the GSK’s product is likely to face a fierce competition in the future.65
GSK3536819A met all endpoints for innovative biologics in clinical trials, demonstrating non-inferiority in the immune response compared to Bexsero and Menveo for all five serogroups.66 This suggests that the effectiveness of the combination vaccine will be similar to the previously approved products, which is estimated at ~70% for MenACWY and 76%-83% for MenB, waning 3-5 years after vaccination.67-69 That innovative biologics also exhibited a safety profile consistent with the licensed vaccines.66 The combination vaccines aim to simplify immunization schedules and improve coverage against life-threatening invasive meningococcal disease among adolescents and young adults. In April 2024, FDA accepted GSK’s application for the MenABCWY vaccine candidate and regulatory decision is set for February 2025.62
mRNA-1010 and mRNA-1083
mRNA-1010 is another mRNA vaccine from Moderna’s stable, which follows the successful application of mRNA technology to produce vaccines against COVID-19 and RSV. It is an investigational quadrivalent seasonal influenza vaccine that aims to address limitations of traditional vaccines, which being prepared in eggs or simian/human cell lines, may contain undesired mutations causing potential strain mismatches. There is also an issue of suboptimal immune response in frail elderly people, who are most vulnerable to severe influenza complications.
The Moderna’s vaccine candidate contains mRNA coding for hemagglutinin (HA) surface glycoproteins from the selected influenza strains recommended by WHO: A/H1N1, A/H1N1, A/H3N2, B/Victoria, and B/Yamagata. Once administered, the mRNA is taken up by host cells, which then produce the HA proteins, eliciting both humoral and cellular immune responses. This approach allows for a more precise immune response and potentially greater efficacy compared to conventional vaccines.
The innovative biologics clinical trials performed so far by Moderna show promising safety and immunogenicity profiles.66,67 In phase 1/2 trial, mRNA-1010 demonstrated superior seroconversion rates for A/H3N2 and A/H1N1 strains compared to standard vaccines, with acceptable tolerability noted across different dosages.70, 71 The Moderna’s candidate was also capable of boosting cellular immunity, as evidenced by the higher frequencies of influenza strain-specific polyfunctional CD4+ lymphocytes. The trials evaluated various dose levels, ultimately focusing on 50 µg as a standard dose that effectively generated desired immune responses without significant safety concerns.
However, Moderna encountered some difficulties upon progressing to Phase 3 trials.72 Although mRNA-1010 was confirmed to be safe and generate superior response to influenza A strains compared with conventional vaccine, it fell short when it comes to the protection against two B strains, recommended by WHO. After this initial stumble, the company decided to go back to blackboard and tweak vaccine formulation so it works equally well for all four viral strains. The pivotal study with the initial innovative biologics mRNA-1010 version was terminated despite being planned to cover an additional influenza season. This courageous move has paid off. The updated version of mRNA-1010 was evaluated in another Phase 3 study, where it met all primary endpoints across all influenza strains.72, 73 Not only did the new vaccine demonstrate non-inferiority against comparator, but it actually produced higher anti-HA (hemagglutinin) titres than the standard seasonal vaccine. Moderna hopes that the US and EU drug agencies would agree to license its product based on the surrogate immunogenicity data, without running the large and costly efficacy trial.
The downside of the new influenza vaccine is certainly its higher reactogenicity compared with the currently available options. Although the trials demonstrated an acceptable safety profile overall, mRNA-1010 recipients complained more frequently about local and systemic reactions to the shot such as injection-site pain, fatigue, myalgia and headache.74 The question now is whether the Moderna’s product will outperform the standard influenza vaccines in terms of protecting the oldest old and immunocompromised. That would justify its widespread use despite the tolerability issues.
The market potential for mRNA-1010 is substantial given the annual demand for effective influenza vaccines globally. For the current season, the US CDC distributed 144.6 million doses of flu vaccine in the United States. Around 45% of the total 18+ population in this country received the shot in the previous year.75 With its innovative approach, superior immunogenicity results and proven technology platform, Moderna will certainly position itself as a strong competitor, especially considering its robust pipeline containing several other influenza vaccine candidates. In addition to mRNA-1010, the company develops vaccines with different HA antigens to offer broader protection against newly-emergent strains (mRNA-1011 and mRNA-1012) and with formulations containing additional influenza antigen called neuraminidase, to boost efficacy.76 Moreover, in June 2024 Moderna announced positive phase 3 trial results for its experimental flu and COVID-19 combination vaccine (mRNA-1083).77 Similarly to mRNA-1010, the two-in-one experimental shot showed that it can elicit a higher immune response compared to the already available separate vaccines.
Unfortunately for Moderna, other companies also explore mRNA technology for flu prevention, including its greatest rival – Pfizer. However, just like Moderna, Pfizer vaccine was also defeated by the rogue influenza B strain.78 In 2020, Pfizer (together with BioNTech) won the race for the first COVID-19 vaccine, being just one week ahead of Moderna. Will the history repeat with flu shots?
***
A short summary of all biologics discussed in our article is presented in Table 2.
| Drug name | Manufacturer | Type | Pharmacological group | Indication |
|---|---|---|---|---|
| Lerodalcibep | LIB Therapeutics | Fusion protein | PCSK9 inhibitor | Secondary prevention of coronary heart disease, hypercholesterolemia |
| Apitegromab (SRK-015) | Scholar Rock | Monospecific antibody | Promyostatin inhibitor | Spinal muscular atrophy (SMA) |
| Depemokimab | GlaxoSmithKline | Monospecific antibody | Anti-IL-5 | Severe, uncontrolled asthma Rhinosinusitis with polyps |
| Clesrovimab | Merck | Monospecific antibody | Anti-RSV fusion protein | Prevention of RSV infection in infants |
| Nipocalimab | Johnson & Johnson | Monospecific antibody | Anti-FcRN | Myasthenia gravis and other immune system disorders |
| Telisotuzumab vedotin (ABBV-399) | Abbvie | Antibody-drug conjugate (ADC) | Anti-c-MET | Non-small cell lung cancer with c-MET overexpression |
| Datopotamab deruxtecan (Dato-DXd) | AstraZeneca | Antibody-drug conjugate (ADC) | Anti-TROP2 | Locally advanced or metastatic EGFR-mutated non-squamous non-small cell lung cancer (NSCLC) |
| Patritumab deruxtecan (HER3-DXd) | Merck | Antibody-drug conjugate (ADC) | Anti-HER3 | Locally advanced or metastatic EGFR-mutated non-squamous non-small cell lung cancer (NSCLC) |
| Mozafancogene autotemcel (Fanca-cel, RP-L102) | Rocket Pharmaceuticals | Gene therapy | Fanconi anemia group A protein stimulant | Fanconi anemia |
| Denecimig (Mim8) | Novo Nordisk | Bispecific antibody | Factor VIII | Hemophilia A |
| Fitusiran | Sanofi Aventis | siRNA | Antithrombin (AT) inhibitor | Hemophilia B |
| Plozasiran | Arrowhead Pharmaceuticals | siRNA | Apolipoprotein C-III inhibitor | Mixed hyperlipidemia |
| MenABCWY (GSK3536819A) | GlaxoSmithKline | Vaccine | Meningococcal group A, B, C, W, Y vaccine | Prevention of meningococcal disease |
| mRNA-1010 mRNA-1083 | Moderna | Vaccine | Influenza (seasonal) vaccine | Influenza prevention |
Prepared by:
Adam Tuszyner
References
- Mullard, Asher. “2024 FDA approvals.” Nature Reviews Drug Discovery (January 02, 2025).
- Coppinger, Caroline, et al. “A comprehensive review of PCSK9 inhibitors.” Journal of Cardiovascular Pharmacology and Therapeutics 27 (2022): 10742484221100107.
- Mercep, Iveta, et al. “PCSK9 inhibition: From effectiveness to cost-effectiveness.” Frontiers in Cardiovascular Medicine 11 (2024): 1339487.
- “LIB Therapeutics Submits a Biologic License Application to FDA for Lerodalcibep for the Treatment of Adults with Elevated LDL-Cholesterol” Business Wire (December 16, 2024).
- Laufs, Ulrich, Matthias Blüher, and Berend Isermann. “Third generation PCSK9-inhibitors.” European Heart Journal 44.40 (2023): 4281-4283.
- Klug, Eric Q., et al. “Efficacy and Safety of Lerodalcibep in Patients With or at High Risk of Cardiovascular Disease: A Randomized Clinical Trial.” JAMA cardiology 9.9 (2024): 800-807.
- Raal, Frederick, et al. “Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia: the LIBerate-HeFH trial.” European Heart Journal 44.40 (2023): 4272-4280.
- Barrett, Doreen, et al. “A randomized phase 1 safety, pharmacokinetic and pharmacodynamic study of the novel myostatin inhibitor apitegromab (SRK-015): a potential treatment for spinal muscular atrophy.” Advances in Therapy 38.6 (2021): 3203-3222.
- Day, John W., et al. “Advances and limitations for the treatment of spinal muscular atrophy.” BMC pediatrics 22.1 (2022): 632.
- “Scholar Rock Reports Apitegromab Meets Primary Endpoint in Phase 3 SAPPHIRE Study in Patients with Spinal Muscular Atrophy (SMA)” Scholar Rock press release (October 7, 2024).
- Crawford, Thomas O., et al. “Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study.” Neurology 102.5 (2024): e209151.
- Scholar Rock press release “Scholar Rock Submits Biologics License Application (BLA) to the U.S. FDA for Apitegromab as a Treatment for Patients with Spinal Muscular Atrophy (SMA)” (January 29, 2025).
- Crescioli, Silvia, et al. “Antibodies to watch in 2025.” mAbs. Vol. 17. No. 1. Taylor & Francis, 2025.
- Nolasco, Santi, and Claudia Crimi. “Depemokimab, the first ultra-long-acting anti-IL-5 monoclonal antibody for severe eosinophilic asthma.” Med 5.12 (2024): 1452-1455.
- Jackson, David J., et al. “Twice-yearly depemokimab in severe asthma with an eosinophilic phenotype.” The New England journal of medicine (2024).
- “Positive results of ANCHOR trials of depemokimab.” GSK press release (October 14, 2024).
- “Clesrovimab (MK-1654): Pediatric Clinical Program Presentation to the Advisory Committee on Immunization Practices.” ACIP meeting (October 23, 2024).
- Madhi, Shabir A., et al. “A phase 1b/2a single ascending dose study of a half-life extended RSV neutralizing antibody, clesrovimab, in healthy preterm and full-term infants.” The Journal of Infectious Diseases (2024): jiae581.
- Ares-Gómez, Sonia, et al. “Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalization for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study.” The Lancet Infectious Diseases (2024).
- Merck News release “Merck Announces FDA Acceptance of Biologics License Application for Clesrovimab, an Investigational Long-Acting Monoclonal Antibody Designed to Protect Infants from RSV Disease During their First RSV Season.” (December 17, 2024).
- Johnson & Johnson press release “Johnson & Johnson seeks first approval of nipocalimab to treat broadest population living with antibody positive generalized myasthenia gravis.” (August 29, 2024).
- Antozzi, Carlo, et al. “Safety and efficacy of nipocalimab in adults with generalized myasthenia gravis (Vivacity-MG3): a phase 3, randomised, double-blind, placebo-controlled study.” The Lancet Neurology 24.2 (2025): 105-116.
- Gottenberg, J. E., et al. “LBA0010 EFFICACY AND SAFETY OF NIPOCALIMAB, AN ANTI-FcRn MONOCLONAL ANTIBODY, IN PRIMARY SJOGREN’S DISEASE: RESULTS FROM A PHASE 2, MULTICENTER, RANDOMIZED, PLACEBO-CONTROLLED, DOUBLE-BLIND STUDY (DAHLIAS).” (2024): 240-240.
- Moise Jr, Kenneth J., et al. “Nipocalimab in early-onset severe hemolytic disease of the fetus and newborn.” New England Journal of Medicine 391.6 (2024): 526-537.
- Strickler, John H., et al. “First-in-human phase I, dose-escalation and-expansion study of telisotuzumab vedotin, an antibody–drug conjugate targeting c-Met, in patients with advanced solid tumors.” Journal of Clinical Oncology 36.33 (2018): 3298-3306.
- Camidge, D. Ross, et al. “Telisotuzumab Vedotin Monotherapy in Patients With Previously Treated c-Met Protein–Overexpressing Advanced Non-Squamous EGFR-Wildtype NSCLC in the Phase 2 LUMINOSITY Trial.” Journal of Clinical Oncology (2024): JCO-24.
- Camidge, D. Ross, et al. “A Phase 1b study of telisotuzumab vedotin in combination with nivolumab in patients with NSCLC.” JTO Clinical and Research Reports 3.1 (2022): 100262.
- Parisi, Claudia, et al. “TROP-2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC).” Cancer Treatment Reviews 118 (2023): 102572.
- Schreiber, Anna R., Michelle Andress, and Jennifer R. Diamond. “Tackling metastatic triple-negative breast cancer with sacituzumab govitecan.” Expert Review of Anticancer Therapy 21.12 (2021): 1303-1311.
- Okajima, Daisuke, et al. “Datopotamab deruxtecan, a novel TROP2-directed antibody–drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells.” Molecular cancer therapeutics 20.12 (2021): 2329-2340.
- Food and Drug Administration (FDA) “FDA approves datopotamab deruxtecan-dlnk for unresectable or metastatic, HR-positive, HER2-negative breast cancer.” (January 17, 2025).
- Bardia, Aditya, et al. “Datopotamab deruxtecan in advanced or metastatic HR+/HER2–and triple-negative breast cancer: results from the phase I TROPION-PanTumor01 study.” Journal of Clinical Oncology (2024): JCO-23.
- Gandullo-Sánchez, Lucía, Alberto Ocaña, and Atanasio Pandiella. “HER3 in cancer: from the bench to the bedside.” Journal of Experimental & Clinical Cancer Research 41.1 (2022): 310.
- Yu, Helena A., et al. “HERTHENA-Lung01: a phase II study of patritumab deruxtecan (HER3-DXd) in previously treated metastatic EGFR-mutated NSCLC.” Future Oncology 19.19 (2023): 1319-1329.
- Merck News release “Patritumab Deruxtecan Demonstrated Statistically Significant Improvement in Progression-Free Survival Versus Doublet Chemotherapy in Patients with Locally Advanced or Metastatic EGFR-Mutated Non-Small Cell Lung Cancer in HERTHENA-Lung02 Phase 3 Trial” (September 17, 2024).
- Krop, Ian E., et al. “Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC).” (2022): 1002-1002.
- Krop, Ian E., et al. “Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3–Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3–Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial.” Journal of Clinical Oncology 41.36 (2023): 5550-5560.
- Food and Drug Administration (FDA) “Approved Cellular and Gene Therapy Products”. (November 21, 2024).
- Segal “Spotlight on Gene Therapies in Q2 2024 Trends” (April 1, 2024).
- Rocket Pharmaceuticals website. Clinical Trials: Fanconi Anemia (FA).
- Rageul, Julie, and Hyungjin Kim. “Fanconi anemia and the underlying causes of genomic instability.” Environmental and molecular mutagenesis 61.7 (2020): 693-708.
- Czechowicz, Agnieszka, et al. “Lentiviral-mediated gene therapy for patients with Fanconi anemia [Group A]: updated results from global RP-L102 clinical trials.” Blood 140.Supplement 1 (2022): 10646-10647.
- BusinessWire “Rocket Pharmaceuticals reports third quarter 2024 financial results and highlights recent progress” (November 7, 2024).
- Napitupulu, Jon “EMA Accepts Rocket Pharma’s RP-L102 Application for Fanconi Anemia Treatment.” Clinical Trial Vanguard (April 3, 2024).
- Rocket Pharmaceuticals website: Clinical Trials.
- Persson, Paula, et al. “Mim8, a novel factor VIIIa mimetic bispecific antibody, shows favorable safety and pharmacokinetics in healthy adults.” Research and Practice in Thrombosis and Haemostasis 7.6 (2023): 102181.
- Bowyer, Annette Elizabeth, et al. “A next generation FVIII mimetic bispecific antibody, Mim8, the impact on non‐factor VIII related haemostasis assays.” Haemophilia 29.6 (2023): 1633-1637.
- Østergaard, Henrik, et al. “A factor VIIIa–mimetic bispecific antibody, Mim8, ameliorates bleeding upon severe vascular challenge in hemophilia A mice.” Blood, The Journal of the American Society of Hematology 138.14 (2021): 1258-1268.
- Bowyer, Annette Elizabeth, Steve Kitchen, and Mirella Ezban. “The effect of a next generation factor VIII mimetic bispecific antibody (Mim8) on assays of factor VIII activity and thrombin generation.” Journal of Thrombosis and Haemostasis 21.3 (2023): 480-487.
- “Novo Nordisk shares positive results from Phase IIIa trial of haemophilia A drug” Clinical Trials Arena (May 14, 2024).
- CDC Hemophilia. Treatment of Hemophilia (November 13, 2024).
- FDA News Release “FDA Approves New Treatment for Hemophilia A or B.” (October 11, 2024).
- Wexler, Marisa “Fitusiran reviewed for hemophilia A, B, with or without inhibitors.” Hemophilia News Today (June 26, 2024).
- Srivastava, Alok, et al. “Fitusiran prophylaxis in people with severe haemophilia A or haemophilia B without inhibitors (ATLAS-A/B): a multicentre, open-label, randomised, phase 3 trial.” The Lancet Haematology 10.5 (2023): e322-e332.
- Manalac, Tristan “5 FDA Decisions to Watch in Q1” BioSpace (January 6, 2025).
- Arrowhead Pharmaceuticals Pipeline. Link: https://arrowheadpharma.com/pipeline/.
- Gaudet, Daniel, et al. “Plozasiran (ARO-APOC3) for severe hypertriglyceridemia: the SHASTA-2 randomized clinical trial.” JAMA cardiology (2024).
- Ballantyne, Christie M., et al. “Plozasiran, an RNA Interference Agent Targeting APOC3, for Mixed Hyperlipidemia.” New England Journal of Medicine (2024).
- Watts, Gerald F., et al. “Plozasiran for managing persistent chylomicronemia and pancreatitis risk.” New England Journal of Medicine (2024).
- “Arrowhead Pharmaceuticals to Advance RNAi-based Plozasiran into Phase 3 CAPITAN Cardiovascular Outcomes Trial.” Arrowhead Pharmaceuticals press release (June 25, 2024).
- “Arrowhead Pharmaceuticals Submits New Drug Application to U.S. FDA for Plozasiran for the Treatment of Familial Chylomicronemia Syndrome.” Arrowhead Pharmaceuticals press release (November 18, 2024).
- GlaxoSmithKline press release “GSK’s 5-in-1 meningococcal ABCWY vaccine candidate accepted for regulatory review by US FDA.” (April 16, 2024).
- Memish, Ziad A., and Abdulrahman A. Alrajhi. “Meningococcal disease.” Neurosciences Journal 7.2 (2002): 77-82.
- CDC Meningococcal Disease: Types of Meningococcal Vaccines (October 24, 2024).
- “Meningococcal (pentavalent) vaccine by Sanofi for Neisseria meningitidis Infections: Likelihood of Approval” Pharmaceutical Technology (December 4, 2024).
- GlaxoSmithKline press release “GSK announces positive pivotal phase III data for 5-in-1 Meningococcal ABCWY vaccine candidate.” (March 14, 2023).
- Castilla, Jesús, et al. “Effectiveness of a meningococcal group B vaccine (4CMenB) in children.” New England Journal of Medicine 388.5 (2023): 427-438.
- Parikh, Sydel R., et al. “Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study.” The Lancet 388.10061 (2016): 2775-2782.
- Cohn, Amanda C., et al. “Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine.” Pediatrics 139.2 (2017).
- Ananworanich, Jintanat, et al. “Safety and immunogenicity of mRNA-1010, an investigational seasonal influenza vaccine, in healthy adults: final results from a phase 1/2 randomized trial.” The Journal of Infectious Diseases (2024): jiae329.
- “Moderna Expands the Field of mRNA Medicine with Positive Clinical Results Across Cancer, Rare Disease, and Infectious Disease.” Moderna News (September 13, 2023).
- Bayer, Max. “After initial stumbles, Moderna finally gains clean phase 3 win for mRNA flu shot.” FiercePharma (September 13, 2023).
- Parkinson, John “Moderna Meets Endpoints with Investigational Influenza Vaccine” Contagion LIVE (September 13, 2023).
- Soens, Mieke, et al. “A phase 3 randomized safety and immunogenicity trial of mRNA-1010 seasonal influenza vaccine in adults.” Vaccine 50 (2025): 126847.
- Centers for Disease Control and Prevention, Flu Vaccination Coverage, United States, 2023–24 Influenza Season (September 20, 2024).
- Russell, Colin A., et al. “Seasonal influenza vaccine performance and the potential benefits of mRNA vaccines.” Human vaccines & immunotherapeutics 20.1 (2024): 2336357.
- Vinluan, Frank. “Moderna’s Flu & Covid-19 Combination Vaccine Succeeds in Phase 3 Clinical Trial.” MedCity News (June 10, 2024).
- Waldron, James. “Pfizer, BioNTech’s combo mRNA shot is latest to be defeated by influenza strain B.” Fierce BioTech (August 16, 2024).
Related resources
Antibodies Development Best Practices for CDMO Collaboration
Drug development, Manufacturing, Monoclonal antibody

UV-VIS Spectrometry for Protein Concentration Analysis: Principles and Applications
Analytics, Biologics, Proteins

Qualitative analysis of Host Cell Proteins using mass spectrometry
Analytics, Drug development, Drug product, Drug substance