Quality as the basis for CDMO partnership success
GMP, Mabion
- Quality assurance and adherence to GMP compliance standards is obviously a critical requirement for any biologics CDMO manufacturing drugs for human use.
- Major quality drivers found in mature systems include management commitment to quality, employee engagement and empowerment, advanced Pharmaceutical Quality System, technical excellence and business continuity.
- A mindset of continuous improvement drives innovation and efficiency, streamlining operations, reducing waste, and lowering operational costs.
Ensuring the highest drug quality is the fundamental obligation of any biopharmaceutical CDMO manufacturing medicines for human use. Companies aiming to consistently provide the finest products and services must cultivate and maintain the so-called quality culture, that is built into all stages of development, manufacturing and control processes.
Quality versus compliance
When considering the definitions of quality and compliance, the latter seems to be more straightforward. Compliance refers to the adherence of a particular organization to regulatory requirements (e.g. GMP), standards set by drug agencies (FDA, EMA or national regulatory bodies) and/or international guidelines (e.g. ICH). To achieve compliance, a company must follow specific protocols, policies, and internal procedures that ensure the products are manufactured in line with the legal and regulatory standards.
Quality, on the other hand, focuses more on the inherent properties of the product or its manufacturing process that guarantee the drug meets all safety and efficacy standards, making it suitable for intended use. Quality culture in an organization refers to the collective mindset and behaviors of its members that prioritize and consistently strive for high quality in all aspects of the organization’s operations. It encompasses the values, beliefs, and practices that support continuous improvement and a commitment to excellence.
Despite the different definitions, compliance can be considered a component of quality, as it ensures that all activities and processes adhere to the predefined legal and regulatory criteria (Figure 1). Both are essential for safe and effective delivery of biopharmaceutical products.

Figure 1 Quality and compliance are distinct yet related concepts.
Biopharmaceutical company
Objective measures of compliance and quality may be invaluable when selecting the right CDMO. Unsurprisingly, compliance appears to be more measurable than quality.
While compliance can be assessed through ordinary inspections or audits, finding appropriate tools for quality assessment presents more challenges.

It is therefore not surprising that not only scientists, but also the FDA experts started investigating this issue, particularly following the severe drug shortages on the US market, which were caused by poor quality practices. This situation prompted FDA to initiate the Quality Management Maturity (QMM) program and develop a dedicated quality metrics tool.2 The prototype assessment protocol is now ready for testing with several companies volunteering for initial evaluations. The establishments demonstrating higher levels of QMM may later benefit from various incentives such as i.e. regulatory flexibility for post-approval changes.
The goals of the FDA program are multifaceted: to foster a quality culture in biopharmaceutical companies, recognize those that advanced quality management practices, identify companies needing system refinements and define the areas for improvement. Ultimately, this initiative aims to minimize the risk of drug shortages. An example of output from the pilot program performed in one of the participating companies is shown in Figure 3.
Earlier analysis of features fostering strong quality culture executed and by the University of St. Galen indicated “top ten” attributes that most strongly differentiate sites based on their quality maturity. 4 Based on comprehensive reviews including the aforementioned, the FDA4 identified five major areas which are considered as indicators of quality maturity: management commitment to quality, business continuity, advanced Pharmaceutical Quality System, technical excellence and employee engagement and empowerment.3 Such analyses demonstrate, that quality is indeed a measurable feature.
Despite differences in understanding and nomenclature under Pharmaceutical Quality System (Quality Assurance vs Quality Unit), both EMA and FDA regulatory frameworks have clear requirements for assuring quality of GMP operations. Additionally, a comprehensive model for a pharmaceutical quality system is specified in the globally harmonized ICH Q10 guideline on pharmaceutical quality system issued in 2015.
Building a quality policy at the CDMO is highlighted as one of the key tools for driving quality practices and ensuring successful project execution.
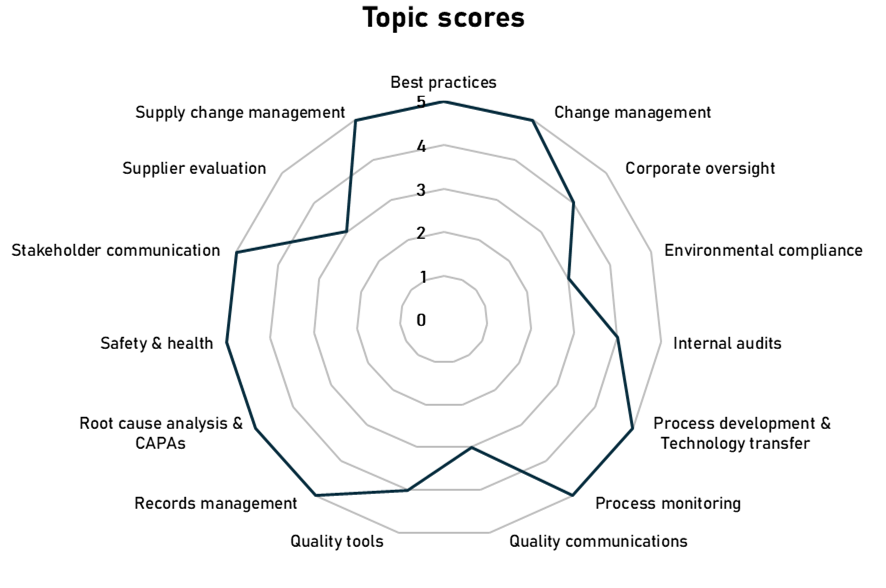
Figure 3 An example of spider plot visualizing company’s performance in different
quality practice areas, as measured during FDA Quality Management Maturity
pilot programs (2020-2022).
Pharmaceutical Quality System and Quality Policy as a foundation of high quality CDMO
Among the five areas used by the FDA for quality maturity assessment, leadership commitment to quality is also emphasized as a major requirement for efficient quality system by the ICH Q10. In addition to other elements, this guideline emphasizes the pivotal role of management in ensuring product quality and regulatory compliance. Similarly, continuous improvement initiatives, driven by comprehensive risk management and quality mindset among employees, significantly contribute to the robustness and effectiveness of company’s operations. Employees who prioritize quality through their attitudes and behaviors are more inclined to seek ways to optimize processes, systems, and practices. This commitment to continuous improvement helps the organization adapt to changes, boost performance and maintain high standards. Other tools for fostering quality culture at pharmaceutical companies are presented in Table 1.
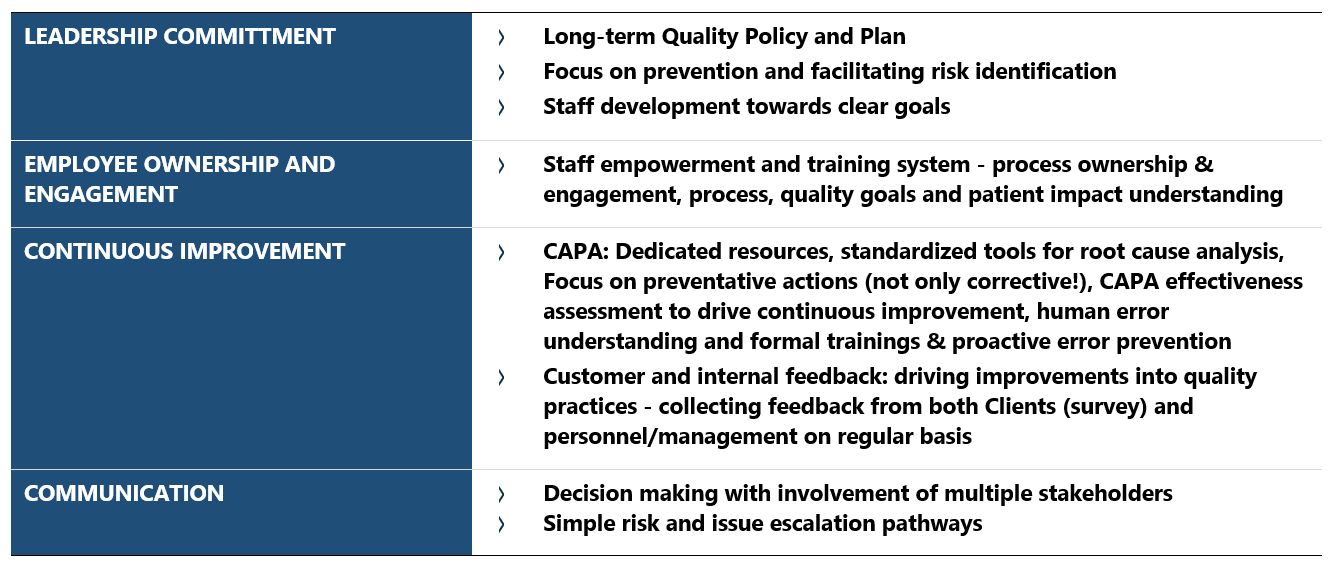
Table 1 Selected tools in focus areas for building mature quality system, based on analysis of Quality Management Maturity FDA/PDA programs.
Quality drives operational excellence of a CDMO
Operational excellence in biologics CMDO is achieved by ensuring that every aspect of the manufacturing process meets the highest standards, thereby enhancing efficiency, reliability and compliance. When a CDMO prioritizes quality, it implements rigorous quality control and quality assurance practices to identify and mitigate risks early in the production cycle. A published benchmarking study provided compelling evidence that adherence to specific quality practices improves manufacturing performance.6
The following practices (Refer to Table 2) were found to correlate with high delivery performance:
- Formal risk management process,
- CAPA effectiveness evaluation,
- Using SPC and 5S tools,
- Identifying bottleneck machines.
It can be inferred from the study, that proactive approach reduces the likelihood of costly errors, which often require rework and may lead to regulatory non-compliance. The cost of errors grows progressively along the stages of drug development and distribution chain. Therefore, implementation of the quality system containing early risk identification and mitigation strategies translates to substantial cost-savings and brings tangible financial benefits. Focusing on quality fosters a culture of continuous improvement, where operational processes are regularly reviewed and optimized for superior performance.

Table 2 Firms that implemented any of the five QMM elements listed in the questions (“Enabler questions”), demonstrated significant improvement in their Delivery KPI.
Another research, conducted by Thermo Fisher Scientific among their Clients, identified the following criteria for determining a CDMO’s quality (in the order of importance)7:
- Supply robustness
- Deviation rates and complaints (CFR definition)
- Resolution times (on-time closure)
- Corrective and preventive action (CAPA) effectiveness
- Activities related to health authorities
- Organization workforce stability
- Frequent communication
- Business consistency.
Unsurprisingly, the list outlines factors that enable to avoid disruption and assure the continuity of supply by the CDMO (supply robustness being on top of the list, demonstrating again that quality is understood as leading to operational excellence)
Conclusions
Manufacturing human drugs by a biopharmaceutical CDMO inherently demands an unwavering commitment to quality. Quality is the foundation of operational excellence, ensuring the production of safe, effective, and regulatory compliant products. Establishment of well-functioning quality system allows CDMOs to increase their efficiency and instill a culture of ongoing improvement that drives innovation. Such endeavor not only avoids a significant financial burden but in the long term results in substantial cost-savings by curtailing the risk of costly errors, while also enhancing operational efficiency and fostering a culture of ongoing improvement and innovation.
References
- Maguire, J., Fisher, A., Harouaka, D., Rakala, N., Lundi, C., Yambot, M., Viehmann, A., Stiber, N., Gonzalez, K., Canida, L., Buhse, L., Kopcha, M. Lessons from CDER’s Quality Management Maturity Pilot Programs. The AAPS Journal 2023; 25(1): 14.
- FDA CDER Quality Management Maturity Program. Available at: https://www.fda.gov/drugs/pharmaceutical-quality-resources/cder-quality-management-maturity (Accessed September 25, 2024).
- US FDA, CDER’s Quality Management Maturity (QMM) Program: Practice Areas and Prototype Assessment Protocol Development, White paper, 2023. Available at: https://www.fda.gov/media/171705/ (Accessed September 25, 2024).
- PDA, How to Measure Quality Management Maturity. Available at: https://www.pda.org/pda-letter-portal/home/full-article/how-to-measure-quality-management-maturity (Accessed September 25, 2024).
- ICH guideline Q10 on pharmaceutical quality system, Step 5, September 2015, EMA/CHMP/ICH/214732/2007.
- Fellows, M., Friedli, T., Li, Y., Maguire, J., Rakala, N., Ritz, M., Bernasconi, M., Seiss, M., Stiber, N., Swatek, M., Viehmann, A. Benchmarking the Quality Practices of Global Pharmaceutical Manufacturing to Advance Supply Chain Resilience. The AAPS Journal 2022; 24(6): 111.
- Transforming CDMO partnerships through a holistic understanding of quality, Thermo Fisher Scientific Whitepaper. Available at: https://www.patheon.com/us/en/insights-resources/whitepapers/transforming-cdmo-partnerships-through-quality.html (Accessed September 25, 2024).
Related resources
Antibodies Development Best Practices for CDMO Collaboration
Drug development, Manufacturing, Monoclonal antibody

UV-VIS Spectrometry for Protein Concentration Analysis: Principles and Applications
Analytics, Biologics, Proteins

Qualitative analysis of Host Cell Proteins using mass spectrometry
Analytics, Drug development, Drug product, Drug substance