Latest trends in the prevention and treatment of cervical cancer
Cancer, Vaccines
- Cervical cancer is one of the few cancers that can be prevented via regular screening (cytology) and vaccination (HPV vaccines). However, despite the widespread availability of preventive measures, hundreds of thousands of women die from it every year.
- Treatment of cervical cancer has improved considerably since the introduction of monoclonal antibodies such as bevacizumab, which targets tumor angiogenesis, or pembrolizumab, which restores natural anti-cancer immunity. Novel solutions, including adoptive T cell therapy (ACT), cervical cancer vaccines or CRISPR/Cas9-based therapies are being developed and have high chances of further boosting the patients’ survival.
- Human papilloma virus (HPV) is responsible for >95% of cervical cancer cases and early administration of HPV vaccine can cut the risk of its development by more than 50%. The incidence of cervical cancer fell considerably in 20-24 year old women in countries, which introduced adolescent vaccination programs.
Background
Every year, cervical cancer is responsible for the death of hundreds of thousands of women, despite the wide availability of effective vaccinations and screening programs. In 2020 its global incidence has been estimated at 13.3 per 100,000 and mortality at 7.2 deaths per 100,000 women, however these numbers vary significantly between the different regions, depending on their economic status 1,2. The incidence in high-income countries is around 9.6 per 100,000 women, while in low-income countries it reaches an extremely high level of 26.7 per 100,000 women, probably due to the lower access to screening and different exposures to risk factors 3. Adding more to these dire statistics, it was calculated that 1 in 111 women in the European Union will develop cervical cancer during their lifetime4.
Cervical cancer is one of the few cancers that has detectable pre-cancerous lesions that can be treated before disease development 5. It is also one of the few cancers that can be prevented through vaccination. This means that a lot can be done to minimize the chances of its development, which is not the case for many other types of cancer. Despite the existence of free-of-charge prevention programs, the participation remains low in many countries – for example in Poland, which offers screening every 3 years, it stays around 24% 6.
At least ninety-five percent of cases of cervical cancer are caused by persistent infection with a commonly encountered, sexually transmitted virus called human papilloma virus (HPV) 7. There are over 200 types of HPV, which differ by their ability to cause malignant transformation 8. Out of these 200 types, only a handful is classified as high-risk – types 16 and 18 clearly stand out, being responsible for around 70% of all cases 9,10. Other pose little or no risk e.g., types 6 and 11 cause benign genital warts rather than cervical dysplasia 10.
Prevention of cervical cancer can be approached by two avenues: HPV vaccines, which prevents the infection with cancer-causing microorganism and screening tests (Pap smear and/or HPV test), which tell if infection has already occurred and if it has led to the development of pre-cancerous lesions 2,3. Detection of the virus and/or premalignant cells gives a chance to thwart the disease at an early stage. Depending on the outcome of Pap test, severe cervical dysplasia on Pap smear is followed by confirmatory biopsy, and confirmed lesions are subject to excision. In high-income regions, cervical screening is a routine procedure, which is open to all women above 20-25 years of age (age eligibility depends on a country) 5.
January is officially recognized as Cervical Cancer Awareness Month. This health campaign aims to promote the knowledge on cervical cancer and popularize the methods of its prevention and treatment. It is then a perfect opportunity to take an in-depth look into the latest developments in this field. In this article we will give a brief overview of new therapies for cervical cancer and progress in vaccination efforts. As a biologics CDMO we will put a special emphasis on novel biologic drugs and their positive impact on patients outcomes and well-being.
Treatment options
Initial therapy of cervical cancer is decided case-by-case, depending on the stage, extent of invasion, histology and involvement of lymph nodes 3. Despite the introduction of more innovative drugs such as monoclonal antibodies, therapeutic approach still involves traditional surgery, chemotherapy, radiotherapy or combination thereof. For early cancer, the recommended therapy ranges from removal of abnormal tissue with fertility preservation to total hysterectomy (excision of entire cervix) with or without removal of the neighboring lymph nodes. For patients who do not qualify for surgery, the only option is radiotherapy. More advanced stages are treated with chemotherapy, chemoradiotherapy with or without the addition of the targeted therapy with monoclonal antibodies. Chemotherapy consists of highly cytotoxic drugs such as cisplatin, carboplatin, topotecan or paclitaxel, that affect also the proliferation of healthy cells 11.
All of these drugs have many unpleasant and sometimes even life-threatening side effects, including neutropenia or neurotoxicity. Nevertheless they are highly effective in reducing the tumor burden and alleviating severe symptoms, that appear at later stages (such as pain or bleeding). Moreover, concurrent administration with radiotherapy significantly improves its outcomes by making malignant cells more vulnerable to the destructive power of radiation 11. As with most other tumors, it has been determined that a combination of differently acting antineoplastic agents significantly prolongs the time to progression and increases patients’ survival. However it is clear that the benefits of more intense regimen must come at the expense of higher toxicity.
Advent of monoclonal antibodies
From the cursory description of available therapies it is apparent that conventional chemotherapy and radiotherapy have many drawbacks, including limited efficacy, substantial recurrence rate and high toxicity. Median survival of patients with advanced cervical cancer treated with cisplatin-based combination is often reported to be less than one year in clinical trials 12.
The introduction of monoclonal antibodies which are characterized by higher specificity and smaller risk of off-target effects, was an incredible breakthrough that considerably improved patients’ outcomes. One of the antibodies used in cervical cancer is bevacizumab, which by binding a signal protein called VEGF (vascular epithelial growth factor) prevents the formation of blood vessels within the tumor (a process called angiogenesis) 3,11. The resultant decrease in oxygen and nutritional supply effectively limits the growth and multiplication of cancer cells. In randomized, controlled trials, the addition of bevacizumab to chemotherapy was shown to increase the survival rate of patients with advanced disease 13,14.
There is also another group of monoclonal antibodies used for cervical cancer. However, because of their distinct and unique mechanism of action, they deserve a separate discussion.
Arrival of immune checkpoint inhibitors
Immune checkpoint inhibitors are one of the latest additions to the cancer treatment arsenal. Speaking shortly, this group consists of monoclonal antibodies that activate the innate immune system to fight cancer cells 11,15. Tumors in general are capable of dampening the immune response by expressing special signal proteins on their surface that deactivate infiltrating lymphocytes and other components of the immune system. In other words, they hide away from body’s natural defense forces, which under normal circumstances would quickly get rid of insubordinate cells. In molecular terms, checkpoint inhibitors bind to one of the proteins involved in the downregulation of immune system – PD-1 (programmed death-1), PD-L1 (programmed death-ligand 1) or CTLA-4 (cytotoxic T-lymphocyte associated protein-4) 15.
The recent decade has been teeming with the successes of various checkpoint inhibitors and their use has quickly expanded from malignant melanoma as a single indication to tens of different cancers, including cervical cancer. Due to the spectacular improvements in patients’ outcomes and unprecedentedly wide anti-tumor activity, these drugs are said to have changed the face of oncology 3.
Pembrolizumab (KEYTRUDA), the most successful immune checkpoint inhibitor, received EMA and FDA approval for use in progressive advanced cervical cancer, either during or after chemotherapy. The antibody works by binding to PD-1 receptor expressed on the surface of lymphocytes, which initiates intracellular cascade leading to the suppression of immune response 15. Tumor cells frequently release its ligand, PD-L1, as well as cytokines that upregulate the formation of PD-1, therefore causing the lymphocytes to abandon their normal function of eliminating cells that are dangerous to the organism. Anti-PD-1 antibodies restore the natural immune response, re-initiating the destruction of cancer cells by surrounding lymphocytes 3.
Expression of PD-L1 in tumors is variable and sometimes even missing, therefore pembrolizumab should be used only in case of PD-L1-positive cervical cancer, diagnosed with a dedicated molecular test. In one of the key trials (“key” is the good word here as the trial was actually named KEYNOTE-826) in patients with persistent, recurrent or metastatic cervical cancer, the results of adding of pembrolizumab to platinum-based chemotherapy +/- bevacizumab were nothing short but phenomenal. Time to the disease progression was much longer in patients assigned to pembrolizumab, especially in those with high expression of PD-L1: 10.4 vs. 8.1 months. Two-year survival in the overall population was 53.0% on pembrolizumab and 41.7% on placebo, meaning that 1 in 9 people who would otherwise be dead, manage to survive 2 years thanks to this antibody 16.
Other popular antibodies from the same family are nivolumab (OPDIVO), cemiplimab and dostarlimab but as of January 2024 they are not yet approved for the treatment of cervical cancer. Also, there is ongoing research on the efficacy of similar mAbs, that act on the opposite side of the immune checkpoint pathway, blocking the PD-1 ligand (PD-L1). In early January this year, The Lancet published the results of Phase III study evaluating one of these drugs – atezolizumab. As observed earlier with pembrolizumab, inhibition of PD-L1 by atezolizumab on the top of conventional therapy slowed cancer progression and increased survival of patients with advanced cervical cancer 17.
New therapies on the horizon
Many new biologic therapies and vaccines for cervical cancer are currently in development. According to the ClinicalTrials.gov, there are more than 1700 ongoing, Phase I-III studies. Some companies try to build on the previously identified and validated molecular targets (e.g. angiogenesis, immune checkpoint), while other explore entirely new strategies that would act on the core pathways of carcinogenesis and overcome the limitations of current treatments.
Some examples of the first approach have been already mentioned: several anti-PD-1 or anti-PD-L1 mAbs such as cemiplimab or dostarlimab are still undergoing clinical evaluation. Given the impressive results of pembrolizumab and atezolizumab trials, they should soon receive regulatory approval.
Although the major successes of immunotherapy in recent years involve monoclonal antibodies, many other types of medications acting on immune systems are being tested for various cancers. One of them is adoptive T cell therapy (ACT), which consists of autologous tumor infiltrating lymphocytes collected from a sample of patient’s tumor and selectively expanded ex vivo to target the cancer cells 13,18,19. After re-infusion, billions of activated T cells seek out the tumor from which they originated and destroy it. ACT is a different type of treatment than CAR-T, which involves genetic engineering of patient’s immune cells. In ACT, T cells are only activated and expanded but not modified in any way. One of the representatives of this group investigated for use in cervical cancer is LN-145, developed by Iovance Biotherapeutics 20. Preliminary research indicates that it could be safely administered alongside the anti-PD-1/PD-L1 monoclonals. Currently, LN-145 is undergoing evaluation in a Phase II study enrolling 189 participants (NCT03108495) 23.
Therapeutic vaccines directed against the HPV-derived oncoproteins E6 and/or E7 are also being investigated for the treatment of cervical cancer and pre-cancerous dysplasia 13,18,19. One example is VGX-3100, which aims to accelerate viral clearance and improve the regression of CIN2/3 lesions associated with HPV-16 or HPV-18. In a recently completed Phase 3 study, 27.6% of VGX-3100 recipients met the primary endpoint versus only 8.7% of counterparts receiving placebo 24. However, despite many years of research, no therapeutic vaccine against cervical cancer has managed to hit the market.
An even more innovative approach involves the use of CRISPR/Cas9 – a revolutionary technique of gene editing, which discovery was recently awarded with Nobel’s prize 13. Owing to its simplicity, affordability and ability to precisely edit any part of the genome, CRISPR/Cas9 is hoped to revolutionize the treatment of many cancers, cervical cancer included. The action of CRISPR/Cas9 system can be described as “genetic scissors”. It uses a synthetic, single guide RNAs (sgRNAs) to generate breaks and remove the selected, complementary parts of genome. The two broken strands are then repaired, either by direct joining of their ends or with the introduction of exogenous DNA sequences. The target of the potential CRISPR/Cas9-based therapy would be highly similar to therapeutic cervical cancer vaccines – excision would involve the genes coding for HPV E6 and/or E7 oncoproteins, which are present in cervical cancer cells 13. This technology showed very promising results in in vitro and animal studies 23,24. Knockout of E6 and E7 proteins triggers senescence in immortalized cell lines and inhibits tumor growth in xenograft formation assays 23. Despite these encouraging data, there is still long way to go before the CRISPR/Cas9 therapy is employed in the clinics.
Prevention better than treatment – deployment of cervical cancer vaccines
This golden rule of medicine is particularly true for cervical cancer. The development of this highly malignant cancer can be easily prevented using two widely available methods: vaccination and screening 3,25. The first one prevents infection with the most oncogenic types of HPV virus, which causes cervical dysplasia and carcinoma, while the second allows for early detection and treatment of the affected lesions before the development of full-blown tumor. It is important to remember that receiving HPV vaccine doesn’t mean that one can forget about regular screening 25. Conversely, doing frequent Pap tests may not be sufficient to ward off the cancer, so even with the availability of free screening programs, vaccination against HPV has still much value. These two pillars of prevention can be imagined as two spider webs, placed one after another – the first one (vaccination) can miss some flies, but with the second one (screening), nearly all flies can be caught.
Each prevention tool has its own limitations. Vaccination doesn’t cover all HPV types and is much less effective when administered in adulthood after the first HPV exposure. On the other hand, screening tests have much less than 100% sensitivity and can miss some dysplastic lesions 26. Only together do they provide solid protection against cervical cancer.
Two major brands of HPV cervical cancer vaccines are currently available on the market: Gardasil (quadrivalent or nonavalent) developed by Merck and Cervarix (bivalent) developed by GSK 27. Both brands contain viral-like particles made of recombinant L1 capsid protein, but they employ different adjuvants – Gardasil is formulated with aluminum phosphate while Cervarix uses AS04, a proprietary adjuvant from GSK 28. Both vaccines include proteins of the most oncogenic HPV types: 16 and 18. Original Gardasil vaccine contains also types 6 and 8, which have low oncogenic potential, but cause genital warts. Therefore, Gardasil, but not Cervarix, has an additional benefit of preventing the formation of genital warts.
Several years ago, Merck released the improved version of their HPV vaccine, which covers additional five types of HPV – Gardasil 9. Together with HPV-6, -8. -16 and -18, the types included in Gardasil 9 are responsible for almost 90% cases of cervical cancer 28. The protection against the covered HPV types approximates 100% with both cervical cancer vaccines and is maintained for many years 29. Multiple observational studies proved beyond any reasonable doubt that HPV vaccination decreases the risk of high-grade cervical dysplasia 30. Obtaining real-world evidence that Gardasil and Cervarix are indeed preventing cancer was much harder and required many years of observation. However, this was finally achieved – recent studies from Sweden, Denmark and England have confirmed that women vaccinated at early age have lower chances of developing cervical cancer than their unvaccinated counterparts 31-33.
Many developed countries introduced school vaccination programs targeting 12-year old girls and initially also older age groups 28,30. The age of 12 was selected in order to ensure protection before sexual debut, that is before the first HPV infection. As the virus is spread primarily by sexual contact and the development of cervical cancer usually takes more than 10 years, the population effect of HPV vaccines can be discerned only after the first vaccinated cohorts reach the adulthood. Countries like Australia, Sweden, Norway, Iceland, Denmark, United Kingdom and Ireland have been vaccinating their children since 2008-2012 35. If indeed HPV vaccine lowers the risk of cervical cancer, the first effects of widespread immunization campaigns should be already observed in these countries. To test this hypothesis, we collected the age-specific data on annual cervical cancer incidence from the national registries operating in the aforementioned countries and calculated the weighted averages for each year 36-40.
We focused on 2005-2021 timeframe to cover the period from right before the vaccine introduction to 10 or more years after, when the vaccinated cohorts have already reached adulthood. Some countries had no data for 2020 and 2021 so we ran two supplementary analyses in which they were removed. The results are displayed in below graphs.
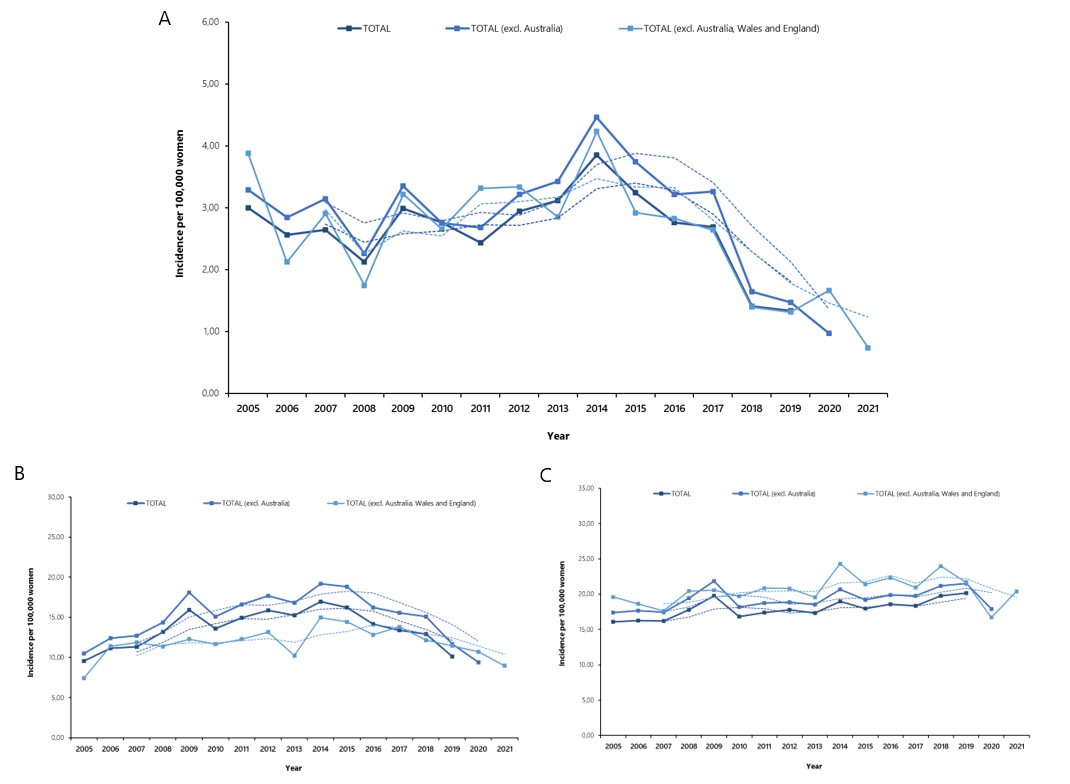
There has been a consistent downward trend in the incidence of cervical cancer in 20-24 year old age group, coinciding with the appearance of vaccinated cohorts. Only a small decline could be observed in 25-29 year old women while the incidence in 30-34 year old group is actually increasing. The slight downward trend in 25-29 year old age group could be due to the “catch-up” immunization efforts or voluntary use of the vaccine. The observed pattern is exactly as one would expect given the established efficacy of cervical cancer vaccines in preventing cervical dysplasia and the timing of immunization campaigns. HPV vaccine has already prevented thousands of cervical cancers and, thanks to the durable protection, it will continue to do so in the future.
Bottomline
Thanks to the spectacular scientific advancements in recent decades, we now have many effective therapies to treat patients with cervical cancer. A combination of standard chemotherapy with anti-angiogenic and immunotherapeutic mAbs allows in many cases to eradicate the disease and let patients live a long, joyful life. However, despite the numerous achievements, the average 5-year survival of cervical cancer patients, regardless of the stage, still remains relatively low, at 67% 41. That’s why it’s important to focus on prevention.
Amazing as it is, cervical cancer is one of the few cancers that can be nearly entirely prevented with the use of cervical cancer vaccines and simple diagnostic tests. Vaccination in adolescence, regular screenings in adulthood and also a healthy lifestyle is all that is needed to keep yourself far away from this malady. Therefore, the key message from Mabion team for the Cervical Cancer Awareness Month is to remember about your next screening visit and vaccinate your children as soon as they turn 12.
Prepared by:
Adam Tuszyner
References
- Singh, Deependra, et al. “Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative.” The Lancet Global Health 11.2 (2023): e197-e206.
- Cohen, Paul A., et al. “Cervical cancer.” The Lancet 393.10167 (2019): 169-182.
- DeVita Jr, Vincent T., Steven A. Rosenberg, and Theodore S. Lawrence. DeVita, Hellman, and Rosenberg’s Cancer: Short Title. Lippincott Williams & Wilkins, 2022.
- European Commission. “Cervical cancer burden in EU-27.” Available at: https://ecis.jrc.ec.europa.eu/pdf/factsheets/cervical_cancer_en-Nov_2021.pdf.
- Cervical Cancer Awareness Month Toolkit. Department of Health.
- Januszek-Michalecka, Lucyna, et al. “Effectiveness of the National Population-Based Cervical Cancer Screening Program in Poland–Outcomes, problems and possible solutions years after implementation.” Annals of Agricultural and Environmental Medicine 20.4 (2013).
- World Health Organization. “Cervical cancer vaccinces.”, 17 November 2023. Available at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- National Cancer Institute (NIH). ”HPV and Cancer”, 18 October 2023. Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer.
- Cancer Council Australia, Clinical Guidelines. “Oncogenic HPV types 16 and/or18.”, 01 July 2022. Available at: https://www.cancer.org.au/clinical-guidelines/cervical-cancer/cervical-cancer-screening/management-of-oncogenic-hpv-test-results/oncogenic-hpv-types-16-and-or-18.
- Bennett, John E., Raphael Dolin, and Martin J. Blaser. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases: 2-Volume Set. Elsevier health sciences, 2019.
- National Cancer Institute (NIH). “Cervical Cancer Treatment by Stage.”, 11 October 2023. Available at: https://www.cancer.gov/types/cervical/treatment/by-stage.
- Hirte, H. W., et al. “Chemotherapy for recurrent, metastatic, or persistent cervical cancer: a systematic review.” International Journal of Gynecological Cancer 17.6 (2007): 1194-1204.
- Burmeister, Carly A., et al. “Cervical cancer therapies: Current challenges and future perspectives.” Tumour Virus Research 13 (2022): 200238.
- Tewari, Krishnansu S., et al. “Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240).” The Lancet 390.10103 (2017): 1654-1663.
- Butterfield, Lisa H., Howard L. Kaufman, and Francesco M. Marincola, eds. Cancer immunotherapy principles and practice. Springer Publishing Company, 2021.
- Colombo, Nicoletta, et al. “Pembrolizumab for persistent, recurrent, or metastatic cervical cancer.” New England Journal of Medicine 385.20 (2021): 1856-1867.
- Oaknin, Ana, et al. “Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial.” The Lancet (2023).
- Monk, Bradley J., et al. “Integration of immunotherapy into treatment of cervical cancer: Recent data and ongoing trials.” Cancer treatment reviews 106 (2022): 102385.
- Ferrall, Louise, et al. “Cervical cancer vaccines and immunotherapy: facts and hopes.” Clinical Cancer Research 27.18 (2021): 4953-4973.
- Rhoades, Alison T. “Oncology Overview: Investigational Autologous TIL Immunotherapy LN-145 for Cervical Cancer.” Pharmacy Times, 1 August 2022.
- Iovance Biotherapeutics, Study of LN-145, Autologous Tumor Infiltrating Lymphocytes in the Treatment of Patients with Cervical Carcinoma. Available at: https://clinicaltrials.gov/study/NCT03108495
- Taylor, Nick. “Inovio’s endpoint switcheroo backfires as phase 3 misses on new measure, hits on old.” Fierce Biotech, 2 March 2023. Available at: https://www.fiercebiotech.com/biotech/inovios-endpoint-switcheroo-backfires-phase-3-misses-new-measure-hits-old.
- Inturi, Raviteja, and Per Jemth. “CRISPR/Cas9-based inactivation of human papillomavirus oncogenes E6 or E7 induces senescence in cervical cancer cells.” Virology 562 (2021): 92-102.
- Gao, Chun, et al. “The application of CRISPR/Cas9 system in cervical carcinogenesis.” Cancer Gene Therapy 29.5 (2022): 466-474.
- National Cancer Institute (NIH). “Cervical Cancer Causes, Risk Factors and Prevention.”, 18 August 2023. Available at: https://www.cancer.gov/types/cervical/causes-risk-prevention.
- Ramírez, Arianis Tatiana, et al. “Performance of cervical cytology and HPV testing for primary cervical cancer screening in Latin America: an analysis within the ESTAMPA study.” The Lancet Regional Health–Americas 26 (2023).
- National Cancer Institute (NIH). “Human Papillomavirus (HPV) Vaccines.”, 25 May 2023. Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet.
- Orenstein, Walter A., et al. Vaccines (8th edition). Elsevier Health Sciences, 2023.
- Kjaer, Susanne K., et al. “Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries.” EClinicalMedicine 23 (2020).
- Herweijer, Eva, et al. “Quadrivalent HPV vaccine effectiveness against high‐grade cervical lesions by age at vaccination: a population‐based study.” International journal of cancer 138.12 (2016): 2867-2874.
- Lei, Jiayao, et al. “HPV vaccination and the risk of invasive cervical cancer (vaccines).” New England Journal of Medicine 383.14 (2020): 1340-1348.
- Falcaro, Milena, et al. “The effects of the national HPV vaccination program in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study.” The Lancet 398.10316 (2021): 2084-2092.
- Kjaer, Susanne K., et al. “Real-world effectiveness of human papillomavirus vaccination against cervical cancer (vaccines).” JNCI: Journal of the National Cancer Institute 113.10 (2021): 1329-1335.
- Palmer, Tim J., et al. “Invasive cervical cancer incidence following bivalent human papillomavirus vaccination: a population-based observational study of age at immunization, dose, and deprivation.” JNCI: Journal of the National Cancer Institute (2024): djad263.
- Loke, Alice Yuen, et al. “The uptake of human papillomavirus vaccination and its associated factors among adolescents: a systematic review.” Journal of primary care & community health 8.4 (2017): 349-362.
- NORDCAN, Association of the Nordic Cancer Registries. Available at: https://nordcan.iarc.fr/en.
- National Cancer Registration and Analysis Service (NCRAS), England. Available at: https://www.cancerdata.nhs.uk/.
- NHS Public Health Wales: Welsh Cancer Intelligence and Surveillance Unit (WCISU). Available at: https://phw.nhs.wales/services-and-teams/welsh-cancer-intelligence-and-surveillance-unit-wcisu/.
- Australian Institute of Heath and Welfare (AIHW). Cancer data in Australia. Available at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/data.
- National Cancer Registry Ireland. Incidence statistics. Available at: https://www.ncri.ie/data/incidence-statistics.
- National Cancer Institute (NIH). “Cervical Cancer Prognosis and Survival Rates” 27 April 2023. Available at: https://www.cancer.gov/types/cervical/survival.
Appendix
Additional information on methods used to construct Figure 1
Age-specific data on cervical cancer incidence for 2005-2021 period were obtained for the following countries/regions and sources 36–40:
- Scandinavia, including Sweden, Norway, Finland, Denmark and Iceland (NORDCAN)
- England (National Cancer Registration and Analysis Service (NCRAS))
- Wales (Welsh Cancer Intelligence and Surveillance Unit (WCISU))
- Scotland (Public Health Scotland)
- Australia (Australian Institute of Health and Warfare)
- Ireland (National Cancer Registry Ireland (NCRI)).
These countries were selected because they introduced adolescent HPV vaccination program in 2008-2012, providing sufficient time for the vaccine effect to be observed. Also, all of them had accessible, high-quality cancer surveillance in place.
Data on incidence were extracted using ICD-10 code for cervical cancer (C53) for 20-24, 25-29 and 30-39 year old age groups.
Weighted averages for the included countries were calculated for each year by multiplying the incidence by the relative population size of a given country and summing the obtained values. The results were then plotted on a linear graph separately for each age group, along with 3-year moving averages.
Incidence for the entire 2005-2021 period could be presented only by excluding Australia, England and Wales, as cancer statistics for recent years were not yet released in these countries (no data for 2020 and 2021 from Australia and no data for 2021 from England and Wales). Data from all included countries were plotted for 2005-2019 period and excl. Australia only for 2005-2020. Trends in incidence remained the same, regardless of the chosen dataset.

If you are interested in our services regarding the development and regulatory aspects of monoclonal antibodies, please don’t hesitate to contact us. Mabion’s specialist offer individualized approach tailored to the biologic and clinical characteristics of the developed drug. Our experience with end-to-end development and manufacturing of biologics gives us an overwhelming advantage over other CDMOs as we perfectly understand the client’s perspective. Click here to obtain more information on our services.
Related resources

Does Your CDMO Have An Analytical Edge?
Analytics, Biosimilars, Mabion

Innovative biologics – expected drug approvals in 2025
Antibody-drug conjugates, Biologics, Bispecific antibody, Clinical trials, EMA, FDA, Monoclonal antibody, Vaccines

Biologics CDMO Solutions for the Future of Therapeutics
Biologics, Drug development, Manufacturing