Top 10 biologics approved in 2023
Biologics, EMA, FDA, Monoclonal antibody, Vaccines

- 2023 witnessed the approval of many innovative biologic drugs that combine the latest scientific discoveries and recently emerging technologies to fight the most dreadful diseases. In total, 18 new biologics were approved by the EMA and 34 by the FDA, an all-time high.
- From this large number we selected and described 10 most important drugs that cover unmet medical needs and have the highest potential to revolutionize medical practice. These include first-ever protein vaccines against RSV, bispecific antibodies for treatment-resistant cancers and CRISPR/Cas9-based gene therapy for sickle cell anemia.
Previous year marked the appearance of a large number of new biologics representing multiple different classes of therapeutics. In total, 18 novel molecules were approved in the EU and 34 in the US, an all-time record.1-3 These include ordinary monoclonal antibodies and protein-based products as well as newly emergent technologies such as bispecific antibodies or drugs employing CRISPR/Cas9 system. Many of them address important medical needs, opening up a way for a treatment of severe genetic disorders or highly resistant cancers.
Inspired by the remarkable scientific progress made over the last year, we decided to review all EMA and FDA approvals in 2023 and produce our own list of top 10 new biologics. Our choice was driven by factors such as the novelty of the mechanism of action, employment of advanced technologies and contribution to public health. After thorough evaluation, we arrived at the following list:
- Elranatamab (ELREXFIO®)
- Glofitamab (COLUMVI®)
- Talquetamab (TALVEY®)
- Delandistrogene moxeparvovec (ELEVIDYS®)
- Examgamglogene autotemcel (CASGEVY®)
- Nirsevimab (BEYFORTUS®)
- Lecanemab (LEQEMBI®)
- Rozanolixizumab (LOARGYS®, RYSTIGGO®)
- Ublituximab (BRIUMVI®)
- RSV vaccines: bivalent, non-adjuvanted (ABRYSVO®) and monovalent, adjuvanted (AREXVY®).
Let’s take a close look on each of these amazing drugs. Embark on a biology approved adventure with Mabion!
Elranatamab (ELREXFIO®)
Elranatamab stands among the three bispecific antibodies included on our list. It belongs to the class of T-cell engagers, which direct T-cells against cancer cells expressing the specific disease-associated antigen.4,5 In the case of elranatamab, the antigen is BCMA (B-cell maturation antigen), implicated in various different hematologic malignancies, with multiple myeloma standing out as the most important. By simultaneous binding to the CD3 receptor and BCMA, elranatamab forms a bridge between T-cells and malignant cells, thereby unleashing the power of patient’s immune system against the latter.5
The approval for the treatment of relapsed or refractory multiple myeloma was granted by both EMA and FDA. In the pivotal Phase 2 study, elranatamab elicited response in 61% of patients, with 35% achieving a complete response.6
Glofitamab (COLUMVI®)
Glofitamab is another bispecific T-cell engager. It binds specifically to CD20 antigen expressed on the surface of B cells and shows considerable activity in the treatment relapsed or refractory diffuse large B-cell lymphoma (DLBCL).7,8 Similarly to other molecules of the same class, glofitamab carries a boxed warning for potentially severe cytokine release syndrome.8 Due to the rapid cytotoxic activity against the lymphoma cells, it also entails a risk of a serious condition called tumor release syndrome.
Despite the serious side effects, glofitamab therapy offers substantial benefits to DLBCL patients. In a pivotal trial, 39% of patients achieved complete response and most of them (78%) were still in remission one year after the treatment initiation.9 Based on these compelling findings, regulatory approvals were granted by both EU and US agencies.
Talquetamab (TALVEY®)
Just as elranatamab, talquetamab is a T-cell engager indicated for the treatment of relapsed or refractory multiple myeloma. However, it binds to a different molecular target – GPRC5D – which is exposed on the surface of malignant cells, contributing to their survival and multiplication. By concurrently engaging CD3 receptor and GPRC5D, talquetamab mediates the killing of myeloma cells by cytotoxic lymphocytes.10
Unsurprisingly, the clinical effects of talquetamab administration closely mirror those of elranatamab, with respect to both efficacy and safety profiles. In a Phase I study involving a cohort of 232 patients – a huge size for an early phase investigation – the response rate was as high as 64%-70%, depending on the administered dosage.11 Furthermore, the observed responses proved to be durable, lasting for a median of 7.8-10.2 months. Unfortunately, the administration of talquetamab was associated with significant reactions, including a cytokine release syndrome which was observed in the majority of subjects.11
Delandistrogene moxeparvovec (ELEVIDYS®)
New FDA drug approvals in 2023 included five gene therapies and one product based on CRISPR-Cas9 technology.3,12 One of the most anticipated gene therapies is delandistrogene moxeparvovec (ELEVIDYS), used for the treatment of Duchenne muscular dystrophy (DMD).13 DMD is a severe, inborn myopathy with a typical onset at the age of 3-5, which results in progressive muscle loss and disability.14 It is caused by a mutation in the gene encoding dystrophin – an important protein present in normal muscle cells, which forms a connection between cytoskeleton and extracellular matrix.15 Without functional dystrophin, muscle fibers become weak and wasted, making the affected person unable to walk or even stand.

The newly approved gene therapy is designed to deliver the key domains of normal dystrophin to myocytes, allowing the muscles to regain their function.13 Delivery is achieved by i.v. injection of adeno-associated virus (AAV) vectors that contain the selected sequences of the dystrophin gene. A robust and sustained expression of Elevidys dystrophin in patients’ muscles follows, leading to the stabilization or improvement in the disease symptoms.16 No untoward consequences have been noted, but the observation period is still too short to ensure absolute confidence.16,17
Elevidys is the first gene therapy for DMD available in the US. However, the initial cost has been set at $3.2 mln, which is beyond the reach of most DMD patients.18 The road to obtain reimbursement from insurance companies is bumpy and may fail to cover all costs. The good news is that there are many different federal and state programs, which can make the gene therapy more accessible for less affluent families.19 It is also fortunate that the treatment consists of a single infusion, expected to last for a lifetime.20 Therefore Elevidys is a one-time expenditure and if it proves to be effective, it may reduce the financial burden associated with other treatments.
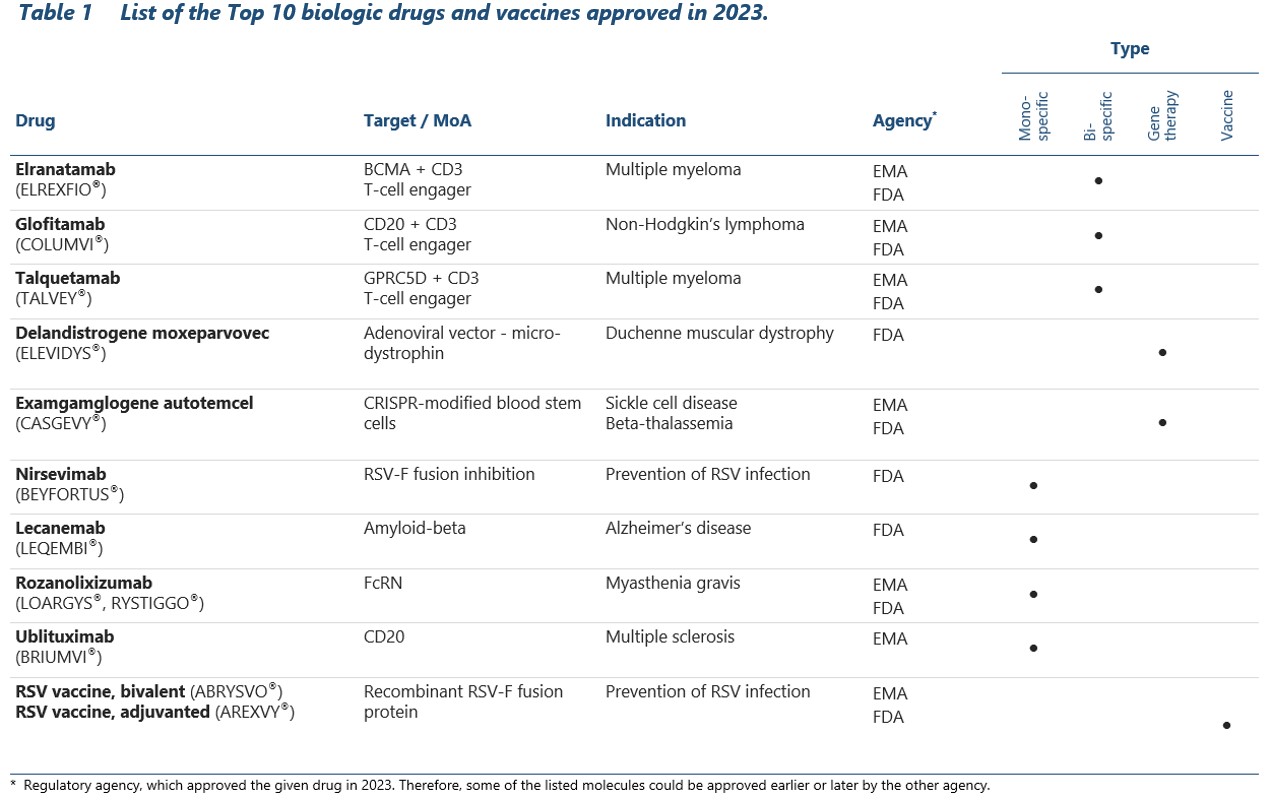
Exagamglogene autotemcel (CASGEVY®)
Last year ended with a significant stride in the field of medical biotechnology. Both UK and US authorities granted approval to the first ever cell therapy utilizing CRISPR/Cas9 gene editing technology – exagamglogene autotemcel (CASGEVY).12,21 This groundbreaking medication targets two hereditary hematological disorders: sickle cell disease and beta thalassemia.21
Both conditions arise from mutations in hemoglobin (beta-globin, specifically), a pivotal protein contained within red blood cells, which delivers oxygen to all body tissues. Characteristic trait of sickle cell anemia is the C-like shape of erythrocytes that lessens their ability to travel through small blood vessels, causing severe pain and increasing the risk of vaso-occlusive episodes. The diminished synthesis or abnormalities in hemoglobin, present in both conditions, result in oxygen deprivation which compromises the functioning of basically every body system.
To prevent severe symptoms and reduce the risk of complications (which may include infections, venous embolism, heart failure and other life-threatening disorders), patients are often forced to undergo frequent transfusions. Bone marrow stem cell transplantation is one of the treatment options, but the procedure carries inherent risks and it remains challenging to find suitable donors. Despite many advances, the management of sickle cell disease still relies mostly on symptomatic treatment – potent analgesics to alleviate the severe pain and antibiotics to reduce the risk of infection.23,24
That’s why Casgevy is viewed as a revolutionary step in the treatment of these two hemoglobinopathies. The drug is made from patient’s own blood stem cells, which are modified in vitro to trigger the production of a special type of hemoglobin called fetal hemoglobin (HbF). Modified cells are administered back to patient, where they improve the function of red blood cells, increase the tissue oxygenation and in consequence obviate the need for regular transfusions.
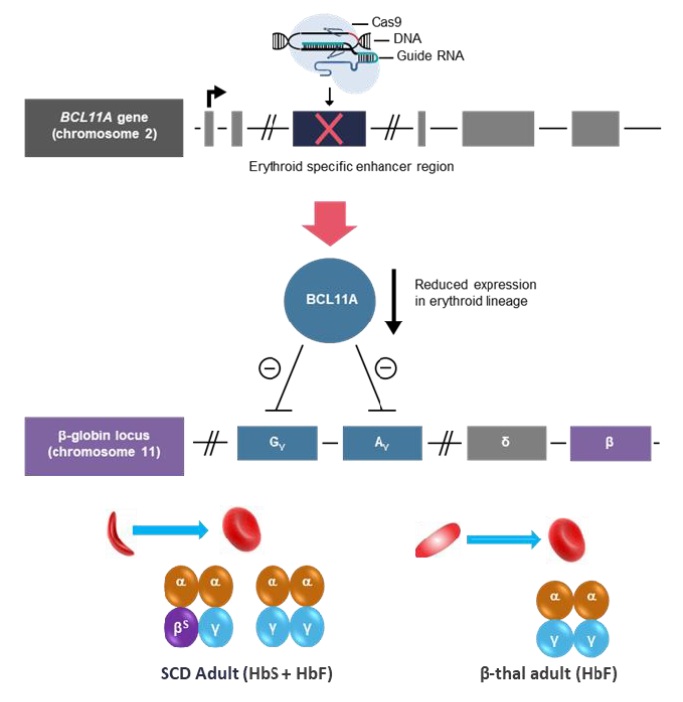
Casgevy approval rested on the outcomes of two efficacy studies, one per each indication.21 The treatment effects were so unequivocal that neither EMA nor FDA required any additional evidence to recommend approval. Nearly all enrolled patients with beta-thalassemia who previously required multiple transfusions per year and had frequent occlusive episodes became essentially free of the disease, at least for a trial duration (median response duration is nearly two years, but the follow-up continues).21,26 Similarly, painful vaso-occlusive episodes that troubled the patients with sickle-cell disease for many years ceased to occur.27 Long-term observation will give answer to the question on whether the treatment effect is permanent or additional dosing is required.
The approval of Casgevy is of huge importance from the public health perspective. Nearly 8 million people in the world are suffering from sickle cell disease, which despite the availability of transfusions, is still characterized by the high mortality rate.28
Casgevy is not the only gene therapy to receive approval for the treatment of sickle cell disease. In December 2023, the FDA licensed also a lentiviral vector-based therapy called lovotibeglogene autotemcel (LYFGENIA®), which causes blood stem cells to produce HbA (T87Q) protein, which is similar to normal adult hemoglobin.29
Nirsevimab (BEYFORTUS®)
The year 2023 was a year of breakthroughs in the prevention and treatment of respiratory syncytial virus (RSV) infections. RSV causes lower respiratory tract infections and is responsible for millions of hospitalizations and thousands of deaths each year, particularly among infants and the elderly.30,31 A new biologic called nirsevimab has a huge potential to reduce this toll.
Nirsevimab is a human monoclonal antibody directed against the RSV fusion protein, expressed on the virus’s surface. By binding to F protein, it impedes the fusion of the virus with cell receptors, thereby preventing viral entry and replication.32
Nirsevimab has been extensively tested in both pre-term and term infants, achieving tremendous reduction in the number of RSV cases and hospitalizations for lower respiratory tract infections. Given its prolonged half-life, a single dose is capable of providing robust protection for up to 6 months. No significant safety issues were observed in clinical trials, with the majority of adverse reactions pertaining to the local injection-related events (redness, swelling and pain at the injection site).33,34
Excellent results of nirsevimab trials prompted some countries, such as Luxembourg, to implement passive immunization campaigns for all newborns. The campaign turned out to be successful as the number and severity of RSV hospitalizations in the following 2023/24 season was substantially reduced and the majority of infants with RSV were not immunized (22 out of 28).35
Lecanemab (LEQEMBI®)
After years of fruitless searching for the new, more effective treatments for Alzheimer’s disease, there’s finally some progress. In 2021, the first effective antibody, aducanumab, has been released for use in the US through the accelerated pathway.36 Previous year marked the appearance of the second mAb – lecanemab.37 Both drugs work by preventing the accumulation of amyloid-β protein, which is suspected to play the key role in the development of Alzheimer’s disease. The two mAbs are currently not available outside the US.
Aducanumab and lecanemab approval came after many years of futile endeavors, during which multiple other amyloid-targeting antibodies were evaluated, each failing to alter the course of Alzheimer’s disease.38 In the clinical trials, lecanemab led to a relatively small improvement in cognition at the expense of significant adverse effects.39 For this reason some professionals warn against its use, especially when the diagnosis is uncertain.40 However, considering the devastating nature of Alzheimer’s disease, not only for the patients themselves but also for their entire families, lecanemab will likely gain much popularity.
Rozanolixizumab (LOARGYS®/ RYSTIGGO®)
Rozanolixizumab is a neonatal Fc receptor blocker (FcRn) approved in 2023 by the FDA for the treatment of myasthenia gravis.41 FcRn prolongs the half-life of IgG antibodies in the circulation, hence it is an attractive therapeutic target for many autoimmune diseases caused by binding of antibodies to self antigens. One of these diseases is myasthenia gravis, in which nicotinic acetylcholine receptors in neuromuscular junctions are blocked and destroyed by patient’s own immune system.
Rozanolixizumab targets the fundamental pathology of myasthenia gravis and its efficacy in the treatment of this condition is indeed remarkable – 61-68% of the drug-treated patients in the pivotal Phase III trial achieved response compared to a mere 28% in the placebo group.41
Ublituximab (BRIUMVI®)
Ublituximab is another member of the large and highly successful class of anti-CD20 monoclonal antibodies (first anti-CD20 mAb was rituximab, approved in the late 90.). It was licensed by the FDA in December 2022 and 5 months later in Europe.42–44 The FDA-approved indications of ublituximab are different types of multiple sclerosis (MS): relapsing-remitting, active secondary progressive and clinically isolated syndromes.43 In Europe, ublituximab was granted only the first indication.42 Its mechanism of action relies on the deep and sustained depletion of B lymphocytes, which are recognized as drivers of MS development and progression.42
Prior to ublituximab approval, several other anti-CD20 antibodies were found useful in the treatment multiple sclerosis. One of them is ocrelizumab, which several years ago received approval from EMA and FDA. Also, there is widespread off-label use of rituximab.45 Therefore the new drug is going to face significant competition, as older products enjoy a cost advantage and established market position.
In clinical trials, ublituximab halved the incidence of MS relapses when compared to teriflunomide – an older non-biologic drug considered to be a standard treatment. However, this high efficacy was counterbalanced by an increased incidence of serious infections.46 Now that the drug is released for use, physicians must carefully weigh the benefits of reducing the MS symptoms against the risk of succumbing to potentially fatal infection.
RSV vaccines (ABRYSVO® and AREXVY®)
Parallel to the approval of nirsevimab, an anti-RSV antibody which we mentioned previously, 2023 welcomed the appearance of two RSV vaccines: Abrysvo (Pfizer) and Arexvy (GSK).47,48 Although both vaccines contain a recombinant F glycoprotein in pre-fusion conformation, their exact composition differs, reflecting the divergent views of their developers on the subject of anti-RSV immunity.49 Abrysvo is a bivalent (covers both RSV type A and B) and non-adjuvanted preparation, while Arexvy is monovalent (only RSV type A) and contains a proprietary adjuvant called AS01E. Presently it seems that these differences have minimal impact on vaccine performance. In large Phase 3 studies conducted in elderly subjects, Abrysvo lowered the risk of RSV-associated lower respiratory tract disease by 67%, while the efficacy of Arexvy was slightly higher, at 83%.50,51 Protection against RSV types A and B was similar for both vaccines.47,48,50-52
In Europe, Pfizer’s vaccine is registered for use in pregnant women between 24 and 36 week of gestation, to achieve protection in infants via maternal transfer of antibodies.52 The risk of severe RSV infection is highest in the first months of life. The competition of Pfizer’s vaccine with nirsevimab will be certainly strong here, as the newly approved mAb appears to be a safer alternative. In contrast to Abrysvo, the vaccine developed by GSK is indicated only for RSV prevention in elderly.53 In United States, Abrysvo received approval in both indications.54 For more information on RSV vaccines, read our recent article “Market welcomes the arrival of RSV vaccines”.
***
Leaving 2023 behind, we are now looking forward to witness the appearance of many new groundbreaking biologics in 2024. This year’s calendar is brimming with potential approvals that can reshape medical practice and offer renewed hope to countless patients. Stay tuned for our next article in which we will delve into biologics that are most likely to make headlines in 2024.
Prepared by:
Adam Tuszyner
References
- European Medicines Agency (EMA). “Human medicines highlights 2023.” Published online 16/01/2024. Link: https://www.ema.europa.eu/en/news/human-medicines-highlights-2023.
- Senior, M. Fresh from the biotech pipeline: record-breaking FDA approvals. Nat Biotechnol (2024).
- Food and Drug Administration (FDA). “2023 Biologic License Application Approvals.” Content current as of 24/01/2024. Link: https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2023-biological-license-application-approvals.
- Tapia-Galisteo, Antonio, Luis Álvarez-Vallina, and Laura Sanz. “Bi-and trispecific immune cell engagers for immunotherapy of hematological malignancies.” Journal of Hematology & Oncology 16.1 (2023): 83.
- Dhillon, Sohita. “Elranatamab: first approval.” Drugs 83.17 (2023): 1621-1627.
- Lesokhin, Alexander M., et al. “Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results.” Nature medicine 29.9 (2023): 2259-2267.
- Shirley, Matt. “Glofitamab: first approval.” Drugs 83.10 (2023): 935-941.
- COLUMVI® Prescribing Information, FDA 2023.
- Dickinson, Michael J., et al. “Glofitamab for relapsed or refractory diffuse large B-cell lymphoma.” New England Journal of Medicine 387.24 (2022): 2220-2231.
- Keam, Susan J. “Talquetamab: first approval.” Drugs 83.15 (2023): 1439-1445.
- Chari, Ajai, et al. “Talquetamab, a T-cell–redirecting GPRC5D bispecific antibody for multiple myeloma.” New England Journal of Medicine 387.24 (2022): 2232-2244.
- Sheridan, Cormac. “The world’s first CRISPR therapy is approved: who will receive it?.” Nature biotechnology 42.1 (2024): 3-4.
- Hoy, Sheridan M. “Delandistrogene Moxeparvovec: first approval.” Drugs 83.14 (2023): 1323-1329.
- Praveen, A., Mathew, G. K. (2019). Medicine: Prep Manual for Undergraduates E-book. Indie: Elsevier Health Sciences.
- Nowak, Kristen J., and Kay E. Davies. “Duchenne muscular dystrophy and dystrophin: Pathogenesis and opportunities for treatment: Third in molecular medicine review series.” EMBO reports 5.9 (2004): 872-876.
- Zaidman, Craig M., et al. “Delandistrogene moxeparvovec gene therapy in ambulatory patients (aged≥ 4 to< 8 years) with Duchenne muscular dystrophy: 1‐year interim results from study SRP‐9001‐103 (ENDEAVOR).” Annals of neurology 94.5 (2023): 955-968.
- Mendell, Jerry R., et al. “Long‐term safety and functional outcomes of delandistrogene moxeparvovec gene therapy in patients with Duchenne muscular dystrophy: a phase 1/2a nonrandomized trial.” Muscle & Nerve 69.1 (2024): 93-98cet (2023).
- Fidler, Ben “Sarepta prices Duchenne gene therapy at $3.2M.” BioPharma Dive (June 22, 2023). Link: https://www.biopharmadive.com/news/sarepta-duchenne-elevidys-price-million-gene-therapy/653720/.
- Usdin, Steve “Medicaid will be the biggest payer for Sarepta’s DMD gene therapy.” Biocentury (June 24, 2023). Link: https://www.biocentury.com/article/648416/medicaid-will-be-biggest-payer-for-sarepta-s-dmd-gene-therapy.
- ELEVIDYS® Prescribing Information, FDA 2023mes, 1 August 2022.
- CASGEVY®. European Public Assessment Report (EPAR).
- Lucarelli, Guido, et al. “Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia.” Cold Spring Harbor perspectives in medicine 2.5 (2012): a011825.
- CDC National Center on Birth Defects and Developmental Disabilities (NCBDDD). Sickle cell disease (SCD). Link: https://www.cdc.gov/ncbddd/sicklecell/index.html.
- CDC National Center on Birth Defects and Developmental Disabilities (NCBDDD). Thalassemia. Link: https://www.cdc.gov/ncbddd/thalassemia/index.html.
- Hoy, Sheridan M. “Exagamglogene autotemcel: first approval.” Molecular Diagnosis & Therapy (2024): 1-7.
- Locatelli, Franco, et al. “Efficacy and safety of a single dose of exagamglogene autotemcel for transfusion-dependent β-thalassemia.” Blood 140.Supplement 1 (2022): 4899-4901.
- Frangoul, Haydar, et al. “Efficacy and safety of a single dose of exagamglogene autotemcel for severe sickle cell disease.” Blood 140.Supplement 1 (2022): 29-31.
- Thomson, Azalea M., et al. “Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021.” The Lancet Haematology 10.8 (2023): e585-e599.
- FDA Vaccines, Blood & Biologics. LYFGENIA®. Link: https://www.fda.gov/vaccines-blood-biologics/lyfgenia.
- CDC. Respiratory Syncytial Virus infection (RSV): RSV Surveillance & Research. Link: https://www.cdc.gov/rsv/research/index.html.
- Li, You, et al. “Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis.” The Lancet 399.10340 (2022): 2047-2064.
- Jorgensen, Sarah CJ. “Nirsevimab: review of pharmacology, antiviral activity and emerging clinical experience for respiratory syncytial virus infection in infants.” Journal of Antimicrobial Chemotherapy 78.5 (2023): 1143-1149.
- Hammitt, Laura L., et al. “Nirsevimab for prevention of RSV in healthy late-preterm and term infants.” New England Journal of Medicine 386.9 (2022): 837-846.
- Griffin, M. Pamela, et al. “Single-dose nirsevimab for prevention of RSV in preterm infants.” New England Journal of Medicine 383.5 (2020): 415-425.
- Ernst, Corinna, et al. “Impact of nirsevimab prophylaxis on pediatric respiratory syncytial virus (RSV)-related hospitalizations during the initial 2023/24 season in Luxembourg.” Eurosurveillance 29.4 (2024): 2400033.
- Planche, Vincent, and Nicolas Villain. “US Food and Drug Administration approval of aducanumab—is amyloid load a valid surrogate end point for Alzheimer disease clinical trials?.” JAMA neurology 78.11 (2021): 1307-1308.
- Hoy, Sheridan M. “Lecanemab: first approval.” Drugs 83.4 (2023): 359-365.
- Zhang, Yun, et al. “Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future.” Signal transduction and targeted therapy 8.1 (2023): 248.
- Van Dyck, Christopher H., et al. “Lecanemab in early Alzheimer’s disease.” New England Journal of Medicine 388.1 (2023): 9-21.
- Kepp, Kasper P., et al. “The anti-amyloid monoclonal antibody lecanemab: 16 cautionary notes.” Journal of Alzheimer’s Disease Preprint (2023): 1-11.
- Bril, Vera, et al. “Safety and efficacy of rozanolixizumab in patients with generalized myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study.” The Lancet Neurology 22.5 (2023): 383-394.
- BRIUMVI®. European Public Assessment Report (EPAR).
- BRIUMVI®. Prescribing Information, FDA 2022.
- TG Therapeutics Press Release “TG Therapeutics Announces FDA Approval of BRIUMVI™ (ublituximab-xiiy).” (December 28, 2022). Link: https://ir.tgtherapeutics.com/news-releases/news-release-details/tg-therapeutics-announces-fda-approval-briumvitm-ublituximab.
- Alcalá, Carmen, et al. “Effectiveness of rituximab vs. ocrelizumab for the treatment of primary progressive multiple sclerosis: a real-world observational study.” Journal of Neurology 269.7 (2022): 3676-3681.
- Steinman, Lawrence, et al. “Ublituximab versus teriflunomide in relapsing multiple sclerosis.” New England Journal of Medicine 387.8 (2022): 704-714.
- European Medicines Agency (EMA). “Abrysvo EPAR – Public Assessment Report.” September 15, 2023.
- European Medicines Agency (EMA). “Arexvy EPAR – Public Assessment Report.” June 16, 2023.
- Che, Ye, et al. “Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine.” Science Translational Medicine 15.693 (2023): eade6422.
- Papi, Alberto, et al. “Respiratory syncytial virus prefusion F protein vaccine in older adults.” New England Journal of Medicine 388.7 (2023): 595-608.
- Walsh, Edward E., et al. “Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults.” New England Journal of Medicine 388.16 (2023): 1465-1477.
- U.S. Food and Drug Administration (FDA). Supporting documents for Abrysvo. Content current as of: September 14, 2023.
- FDA Highlights of Prescribing Information. Arexvy (2023).
- FDA Highlights of Prescribing Information. Abrysvo (2023).

If you are interested in our services regarding the development and regulatory aspects of monoclonal antibodies, please don’t hesitate to contact us. Mabion’s specialist offer individualized approach tailored to the biologic and clinical characteristics of the developed drug. Our experience with end-to-end development and manufacturing of biologics gives us an overwhelming advantage over other CDMOs as we perfectly understand the client’s perspective. Click here to obtain more information on our services.
Related resources
Antibodies Development Best Practices for CDMO Collaboration
Drug development, Manufacturing, Monoclonal antibody

UV-VIS Spectrometry for Protein Concentration Analysis: Principles and Applications
Analytics, Biologics, Proteins

Qualitative analysis of Host Cell Proteins using mass spectrometry
Analytics, Drug development, Drug product, Drug substance