Effectiveness of 2023-24 season COVID vaccines: First estimates are in
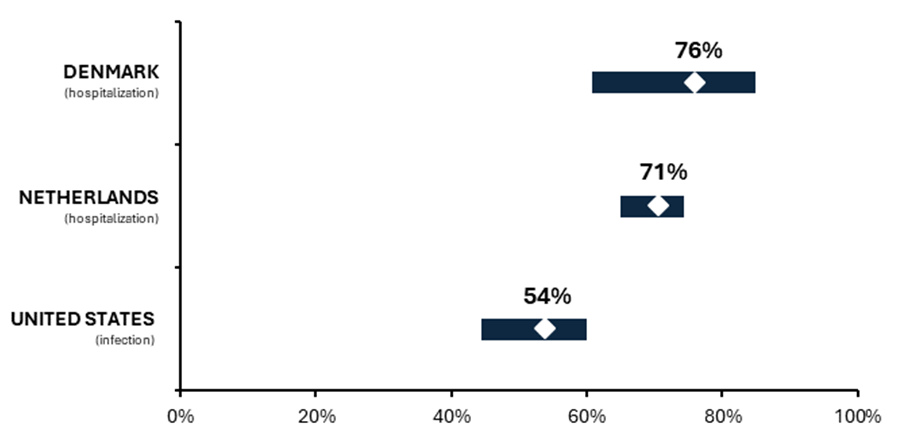
- First studies estimating the effectiveness of updated COVID-19 vaccines have been published. Effectiveness against hospitalization was calculated at 70.7%-76.1% and against symptomatic SARS-CoV-2 infection at 54%.
- These estimates were derived by comparing the outcomes of re-vaccinated patients with patients, who in majority received the previous vaccinations. The actual effectiveness in comparison to the unvaccinated population is probably much higher.
- The available data cover only a short period of time after the booster receipt. Effectiveness is likely to wane with the increasing follow-up and ongoing evolution of the virus.
First three studies from Denmark, Netherlands and United States indicate high effectiveness of the updated, 2023-2024 season COVID vaccines. Protection against hospitalization ranged from 70.7% in Netherlands to 76.1% in Denmark (see the graph below). Protection against severe COVID, i.e. intensive care unit admission, could be estimated only using the Dutch data, and the value was similar – 73.3%.
In Danish and US studies, the effectiveness was estimated by comparing re-vaccinated patients against patients who have not yet received the adapted vaccine, but who in majority were vaccinated with the previous doses of monovalent or bivalent preparations. This means that the reported values are not absolute but incremental, and the actual effectiveness compared with the unvaccinated population is probably much higher.
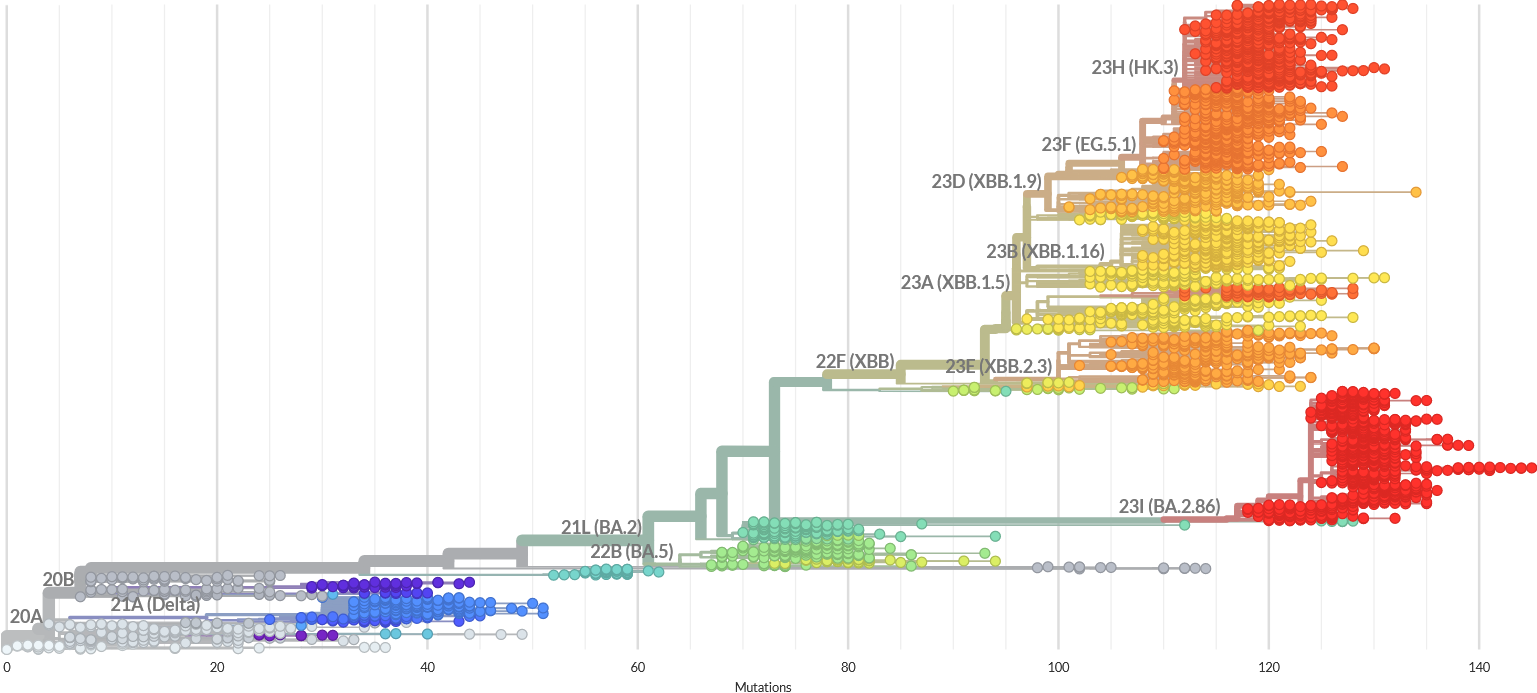
Adaptation of the already registered COVID-19 vaccines was necessary due to the high divergence of the newer SARS-CoV-2 variants such as XBB.1.5. or BA.2.86 (see the figure below). Comparing with the original Wuhan virus, recently submitted sequences show up to 132 nucleotide changes and 101 gaps, most of them involving the region coding for spike protein. Such a fast evolution makes people with past vaccination or infection susceptible to the new variants. In addition, antibodies generated by the existing COVID-19 vaccines wane rapidly, so annual re-vaccination is needed to prevent the infection.
Fortunately, cellular immunity persists longer so even the previously received doses are highly effective at reducing the risk of severe outcomes. Nevertheless, adapted vaccines increase this protection by another 70%, further minimizing the risk of hospital and ICU admissions.
A major limitation of these preliminary studies is their short duration. For example, the Danish study followed re-vaccinated patients for only 10 days after the index date. The US study is much better in this respect, giving 52 days of follow-up. Another issues are insufficient data regarding the most severe outcomes such as COVID-related death and no information on brand-specific effectiveness.
Waning immunity and continuing virus evolution will likely decrease the end-of-season effectiveness of updated vaccines. So far, these phenomena are not observed in the published data, even in the timeframe of 60-119 days since last dose, which was available in the US study (reported effectiveness of 49%). Another 1-2 months are needed to obtain more reliable estimates.
Prepared by:
References
- Hansen, Christian Holm, et al. “Short-term effectiveness of the XBB. 1.5 updated COVID-19 vaccine against hospitalization in Denmark: a national cohort study.” The Lancet Infectious Diseases (2024).
- van Werkhoven, C. Henri, et al. “Early COVID-19 vaccine effectiveness of XBB. 1.5 vaccine against hospitalization and admission to intensive care, the Netherlands, 9 October to 5 December 2023.” Eurosurveillance 29.1 (2024): 2300703.
- Link-Gelles, Ruth. “Early Estimates of Updated 2023–2024 (Monovalent XBB. 1.5) COVID-19 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Attributable to Co-Circulating Omicron Variants Among Immunocompetent Adults—Increasing Community Access to Testing Program, United States, September 2023–January 2024.” MMWR. Morbidity and Mortality Weekly Report 73 (2024).
- Keam, Susan J. “Teplizumab: first approval.” Drugs 83.5 (2023): 439-445.
- Nextstrain.org. Genomic epidemiology of SARS-CoV-2 with subsampling focused globally over the past 6 months. Link: https://nextstrain.org/ncov/gisaid/global/6m?m=div.