First-in-class IL-15 superagonist wins FDA approval
- Anktiva®, a first-in-class immunotherapeutic agent mimicking the action of interleukin-15 (IL-15), has received FDA approval in the treatment of non-muscle invasive bladder cancer (NMIBC).
- The new drug features a highly unique structure, being a fusion protein of IL-15 receptor domain (IL-15RαSu) and Fc part of IgG1 complexed with mutated IL-15. It works by activating NK cells and cytotoxic lymphocytes against cancer cells.
- Clinical trials demonstrated that the broad activation of cancer-killing cells by Ankiva® results in complete and durable responses in the majority of treated NMIBC patients (62%). Almost half of the responders continues in remission after two years of follow-up. The drug is currently tested in treatment of other solid tumors as well as HIV infection.
Another class of anti-tumor biologic has just arrived on the scene, with the recent FDA approval of a groundbreaking immunotherapeutic drug, Anktiva® (also known as N-803 or nogapendekin alfa inbakicept). Anktiva®, developed by US bio-tech start-up ImmunityBio, is the first and currently the only available superagonist of interleukin-15 (IL-15), a potent immunostimulatory cytokine. In pharmacology, the term “superagonist” is used to describe a drug that is capable of producing a maximal response higher than the endogenous receptor ligand. Indeed, Anktiva® is reported to be 4-5 times more active than the natural cytokine from which it originates. The FDA approved Anktiva® for the treatment of non-muscle invasive bladder cancer (NMIBC) not responding to the standard BCG vaccine therapy – a narrow indication, which is likely to be expanded over the next few years.
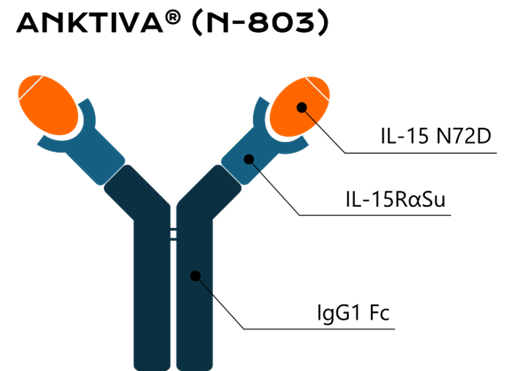
Anktiva® has a remarkably unique structure, unlike any of the biologics available on the market. It consists of a dimeric complex of a fusion protein, containing IL-15 receptor Sushi domain (IL-15RαSu) and Fc part of IgG1 antibody, and mutated IL-15 (see graphic above). This sophisticated design stabilizes the inherently unstable cytokine, prolonging its half-life and enabling clinical use. Moreover, the mutation introduced to IL-15 i.e., substitution of asparagine to aspartic acid at position 72, enhances its potency by increasing the binding to the IL-2 receptor.
Anktiva® reproduces the function of the IL-15, albeit with enhanced and prolonged activity compared to the natural cytokine. Its mechanism of action resembles widely used immune checkpoint inhibitors, that is activation of the key components of the immune system to target cancer cells. However, the spectrum of Ankiva’s activity is much broader than that of older drugs such as pembrolizumab or nivolumab, as it not only activates CD8+ lymphocytes but also engages NK cells, another important player in body’s anti-tumor response. Activated CD8+ lymphocytes and NK cells infiltrate the tumor, recognize the cancer-associated antigens and initiate a strong cytotoxic response, which leads to cancer cell death.
The joint activation of killer T lymphocytes and NK cells by Anktiva® proved to be exceptionally effective in the treatment of advanced and BCG-unresponsive bladder cancer. In a clinical trial leading to FDA approval, the IL-15 superagonist induced complete responses (defined as disappearance of all target lesions) in 62 percent of the treated patients. Moreover, the median duration of those responses exceeded 47 months, with 58 percent of responders in remission for at least 12 months and 40 percent for at least 24 months. These results, obtained in a difficult-to-treat patient population provided unequivocal evidence of drug efficacy and formed the basis for FDA approval.
ImmunityBio, the innovator behind Anktiva®, focuses on the development of new treatments for cancers and infectious diseases that effectively cure the disease while also protecting patient’s immune system. The company has already announced the completion of manufacturing of drug substance sufficient for 170,000 doses and the product is set to become available in the US this month. Meanwhile, the development of Anktiva® in other indications continues unabated. Multiple trials are currently underway testing the IL-15 superagonist in other solid tumors such as lung cancer, colorectal cancer or ovarian cancer, as well as hematological malignancies. Anktiva® is also evaluated in the treatment of acute HIV infection, in which it is hoped to reduce the viral persistence in lymphoid tissues. The successful registration in bladder cancer will probably soon be followed by non-small lung cancer – the company has just reported improved survival among patients treated with Ankiva® in combination with immune checkpoint inhibitors. A meeting with the FDA to discuss the regulatory filing is scheduled for June.
Prepared by:
Sources and further reading
- Manalac, Tristan, “ImmunityBio Wins FDA Approval for First IL-15 Superagonist for NMIBC.” BioSpace (April 23, 2024). Link: https://www.biospace.com/article/immunitybio-wins-fda-approval-for-first-il-15-superagonist-for-nmibc.
- ImmunityBio press release “ImmunityBio Announces FDA Approval of ANKTIVA®, First-in-Class IL-15 Receptor Agonist for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer” (April 22, 2024). Link: https://immunitybio.com/immunitybio-announces-fda-approval-of-anktiva-first-in-class-il-15-receptor-agonist-for-bcg-unresponsive-non-muscle-invasive-bladder-cancer/.
- ImmunityBio press release “ImmunityBio Completes GMP Drug Substance Manufacturing Sufficient for 170,000 Doses of ANKTIVA®” (May 7, 2024). Link: https://immunitybio.com/immunitybio-completes-gmp-drug-substance-manufacturing-sufficient-for-170000-doses-of-anktiva/.
- ImmunityBio press release “ImmunityBio Announces Positive Overall Survival Results of Anktiva Combined With Checkpoint Inhibitors in Non-Small Cell Lung Cancer; Meeting Scheduled with FDA to Discuss Registration Path for ANKTIVA in Lung Cancer.” (April 24, 2024). Link: https://immunitybio.com/immunitybio-announces-positive-overall-survival-results-of-anktiva-combined-with-checkpoint-inhibitors-in-non-small-cell-lung-cancer-meeting-scheduled-with-fda-to-discuss-registration-path-for-anktiv/