The power of antibody-drug conjugates revealed in recent cancer trials
- A new treatment combination containing antibody-drug conjugate called enfortumab vedotin, doubled the survival of patients with metastatic bladder cancer compared with the standard chemotherapy.
- This spectacular result together with the positive findings of other recently completed studies underscores the power of antibody-drug conjugates in treating advanced cancers.
A flurry of new studies published this year report the successful treatment of various cancers with antibody-drug conjugates (ADCs), which work by navigating the cytotoxic molecules straight to the malignant cells. The latest Phase III clinical trial of an ADC called enfortumab vedotin in patients with advanced bladder cancer provides yet more evidence that this technology can save lives.
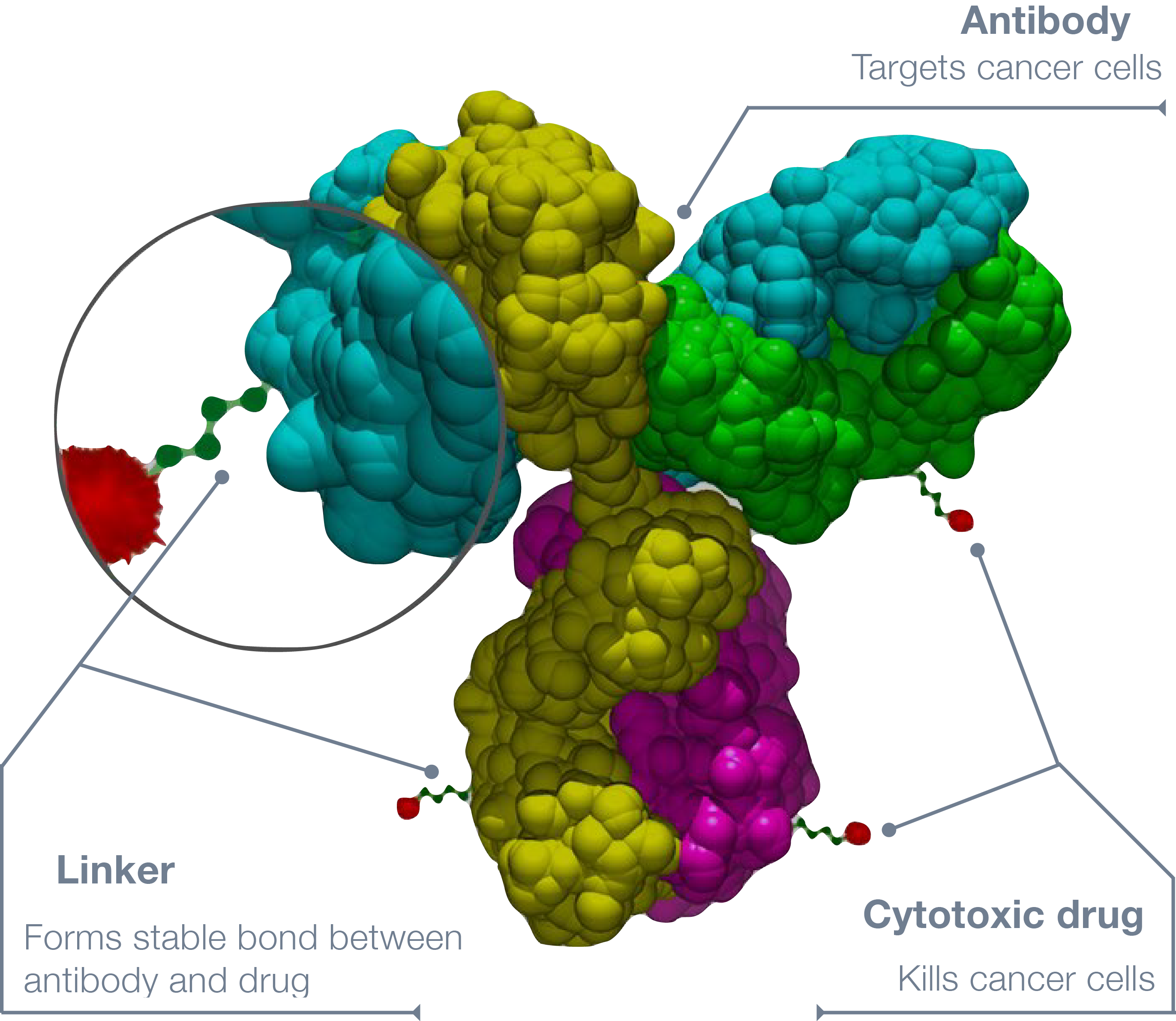
Antibody-drug conjugates such as enfortumab vedotin allow for efficient and highly-targeted delivery of cytotoxic molecules to malignant cells.
Enfortumab vedotin, consists of a monoclonal antibody targeting nectin-4, an abundantly expressed protein responsible for cellular adhesion, and monomethyl auristatin E (MMAE), a highly toxic molecule which disrupts cell division. Due to its extreme toxicity to healthy cells, MMAE has to be conjugated to mAb, in order to ensure highly efficient and targeted delivery to cancer cells. This molecule is also present in other ADCs such as brentuximab vedotin or polatuzumab vedotin.
In the reported trial, enfortumab vedotin was administered on the top of pembrolizumab therapy, a monoclonal antibody belonging to the successful class of immune checkpoint inhibitors, which combat malignant cells by restoring intrinsic anti-cancer immunity. The results of applying this antibody cocktail were nothing short of amazing, with the median survival time of patients extended from 16 to 31.5 months. Typically, the trials of cancer drugs show only a few months or even weeks of added survival, and it is very uncommon to see the one that doubles it. The reported trial involved over 880 people, who were assigned to receive either the new treatment or conventional chemotherapy, so the benefit of enfortumab vedotin-pembrolizumab combination could be documented with appropriate statistical power and precision.
The advent of antibody-drug conjugates is celebrated particularly by oncologists who care everyday for patients with incurable, terminal cancers such as metastatic bladder cancer. Previous therapeutic options, consisting of conventional cytotoxic drugs, had limited effectiveness, but quite substantial side effects. Decades of research spent on finding the more effective and safe therapy for bladder cancer have failed miserably… until this moment.
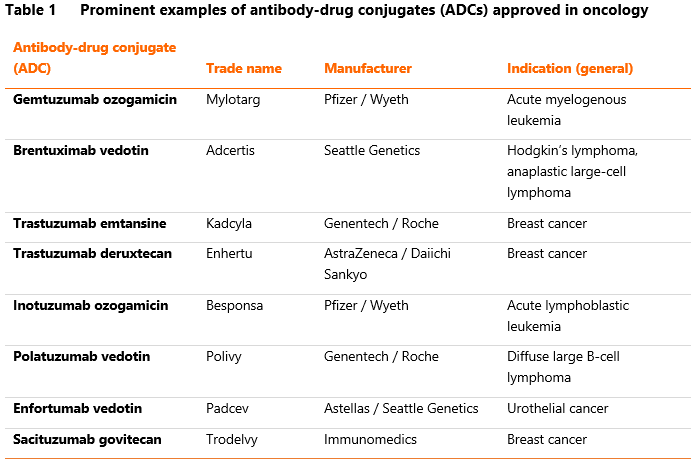
The survival benefit observed in bladder cancer is only one of many successes reported for ADCs in recent years. Polatuzumab vedotin, targeting CD79 receptor, was found to increase the time to progression among previously untreated patients with diffuse large B-cell lymphoma, when added to the standard chemoimmunotherapeutic regimen. Trastuzumab deruxtecan, a drug-conjugated version of the famous anti-HER2 antibody, showed high activity in patients with pre-treated breast cancer and gastric cancer. Enfortumab vedotin itself has been previously evaluated in urothelial cancer, in which it also led to improved progression-free and overall survival.
However beneficial in terms of efficacy, antibody-drug conjugates are still burdened by many toxic reactions, which originate not only from antibody part itself but also from the conjugated molecule. Although it has been hypothesized that conjugation would prevent most of the off-target side effects, this turned out to be not the case. Some patients treated with ADCs may experience hepatotoxicity, interstitial lung disease, peripheral neuropathy, neutropenia or thrombocytopenia, all of which are typical for the employed payloads rather than monoclonal antibodies.
Despite the increased toxicity, for most of ADCs the balance of benefits and risks is clearly shifted towards the former. One thing that we can be certain of is that the story of ADCs is still unfolding, with more positive news to come as other molecules complete the late-phase clinical trials.
References
- Ledford, Heidi. “Cancer trial results show power of weaponized antibodies.” Nature (2023).The Center for Biosimilars (November 6, 2023).
- Powles, Thomas MD. “Enfortumab Vedotin/Pembrolizumab Shows Encouraging Survival Data in mUC.” Targeted Oncology (October 22, 2023). Link: https://www.targetedonc.com/view/enfortumab-vedotin-pembrolizumab-shows-encouraging-survival-data-in-muc
- Cortés, Javier, et al. “Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer.” New England Journal of Medicine 386.12 (2022): 1143-1154.
- Shitara, Kohei, et al. “Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer.” New England Journal of Medicine 382.25 (2020): 2419-2430.
- Tilly, Hervé, et al. “Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma.” New England Journal of Medicine 386.4 (2022): 351-363.
- Powles, Thomas, et al. “Enfortumab vedotin in previously treated advanced urothelial carcinoma.” New England Journal of Medicine 384.12 (2021): 1125-1135.
- Fu, Zhiwen, et al. “Antibody drug conjugate: the “biological missile” for targeted cancer therapy.” Signal transduction and targeted therapy 7.1 (2022): 93.